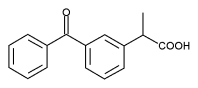
FORT LAUDERDALE, Fla. and CORONA, Calif.--(BUSINESS WIRE)--March 30, 1999--Andrx Corporation (Nasdaq:ADRX) and Watson Pharmaceuticals, Inc. (NYSE:WPI) today announced that the United States Food and Drug Administration (FDA) has approved an Abbreviated New Drug Application (ANDA) submitted by their joint venture, ANCIRC Pharmaceuticals for bioequivalent versions of Oruvail(R).
Marketed by the Wyeth Ayerst division of American Home Products, Inc., Oruvail(R) (Ketoprofen Extended-release Capsules) is used in the treatment of arthritis. This product was developed by Andrx and will also be manufactured and marketed by Andrx on behalf of ANCIRC.
Andrx is engaged in the formulation and commercialization of oral
controlled-release pharmaceuticals utilizing its proprietary drug delivery technologies. In its ANDA program, Andrx is developing generic versions of selected high sales volume controlled-release brand name pharmaceuticals. In its NDA program, Andrx is developing its own brand name formulations of certain existing drugs that it believes may be improved by the application of the Andrx' drug delivery technologies. Andrx also distributes pharmaceutical products manufactured by third parties and is developing Internet based software applications to improve the provision of healthcare.
Watson Pharmaceuticals, Inc., headquartered in Corona, CA, is engaged in the development, manufacture and sale of proprietary and off-patent pharmaceutical products. Watson pursues a strategy of generating revenue through established proprietary and off-patent businesses, capitalizing on its proven ability to support the development of these drugs in the therapeutic areas of primary care, women's health, dermatology and neurology/psychiatry.
Forward-looking statements (statements which are not historical facts) in this release are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995.
Contacts: Elliot F. Hahn, Ph.D., President of Andrx Corporation, 4001 S.W. 47th Avenue, Fort Lauderdale, Florida 33314, 954-584-0300. This release and additional information about Andrx Corporation are also available on the Internet at: http://www.Andrx.com. Sara Swee, Corporate Communications of Watson Pharmaceuticals, Inc., 909-270-1400. This and past press releases of Watson Pharmaceuticals, Inc. are available at Watson's web site at www.watsonpharm.com. In addition, press releases are available through PR Newswire's Company News On-Call fax service at 800-758-5804, extension 112856, and at www.prnewswire,com.
COPYRIGHT 1999 Business Wire
COPYRIGHT 2000 Gale Group