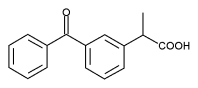
PHILADELPHIA--(BUSINESS WIRE)--Feb. 15, 1995--Wyeth-Ayerst Laboratories, Division of American Home Products Corporation (NYSE:AHP), today announced market clearance for ORUVAIL(R) (ketoprofen) 100 and 150 mg extended-release capsules.
This adds valuable dosing flexibility to Oruvail 200 mg extended release capsules, the first nonsteroidal anti-inflammatory drug in an extended-release, once-a-day, pH-dependent dosage form.
ORUVAIL is indicated for the management of the signs and symptoms of osteoarthritis and rheumatoid arthritis, conditions that affect 15.8 million and 2.1 million Americans respectively. Since it became available in the United States in the fall of 1993, physicians have written over 1 million prescriptions for ORUVAIL. The new 100/150 mg capsules will allow for even broader use of Oruvail among elderly and renally impaired patients.
Wyeth-Ayerst is a major research-oriented pharmaceutical company with leading products in the areas of women's health care, cardiovascular and metabolic disease therapies, central nervous system drugs, anti-inflammatory agents, vaccines, infant nutritionals and generic pharmaceuticals. American Home Products Corporation ranks among the top three companies in the world in sales of pharmaceuticals and health-care products. It is also a leader in agricultural products, animal health care, medical devices and food products.
CONTACT: Wyeth-Ayerst Laboratories, St. Davids, Pa.
Audrey Ashby, 610/971-5823
or Jeff Hoyak, 610/971-5814
COPYRIGHT 1995 Business Wire
COPYRIGHT 2004 Gale Group