Study objective: Weight loss is a common complication of COPD, associated with negative outcomes. Weight restoration has been associated with improved outcomes. The effects of oxandrolone, an adjunct to help restore weight, were evaluated in patients with COPD.
Design: Prospective, open-label, 4-month clinical trial.
Setting: Twenty-five community-based pulmonary practices throughout the United States.
Patients: A primary pulmonary diagnosis of moderate-to-severe COPD as defined by FE[V.sub.1] < 50% of predicted and FE[V.sub.1]/FVC ratio < 0.7, along with significant involuntary weight loss (weight [less than or equal to] 90% ideal body weight).
Interventions: Oral oxandrolone, 10 mg bid.
Measurements and results: Body weight, body composition (bioelectric impedance analysis), spirometry, and 6-min walking distance were measured. Data for 82 patients at 2 months and 55 patients at 4 months are presented. At month 2, 88% of patients had gained a mean [+ or -] SD of 6.0 [+ or -] 4.36 lb (p < 0.05) and 12% had lost a mean of 1.7 [+ or -] 2.15 lb (not statistically significant [NS]). At month 4, 84% had gained a mean of 6.0 [+ or -] 5.83 lb (p < 0.05) and 16% had lost a mean of 1.8 [+ or -] 1.74 lb (NS). Month 4 bioelectric impedance analysis showed the weight to be primarily lean tissue, with a mean increase in body cell mass of 3 [+ or -] 2.6 lb (p < 0.05), and a mean increase in fat of 1.2 [+ or -] 4.6 lb (NS).
Conclusions: Oxandrolone is an effective adjunct to facilitate weight restoration in patients with COPD-associated weight loss. Weight gain is primarily lean body mass. Oxandrolone was relatively well tolerated and, therefore, should be a consideration in the comprehensive management of patients with COPD and weight loss.
Key words: anabolic steroids; body cell mass; body composition; COPD; involuntary weight loss; lean body mass; 6-min walking distance; oxandrolone
Abbreviations: ALT = alanine aminotransferase; AST = aspartate aminotransferase; BCM = body cell mass; BMI = body mass index; IBW = ideal body weight; LBM = lean body mass; NS = not statistically significant; PEmax = maximum expiratory pressure; PImax = maximum inspiratory pressure; QOL = quality of life; 6MWD = 6-min walking distance; VAS visual analog scale
**********
Involuntary weight loss is a common complication of COPD, and is associated with negative outcomes independent of the degree of airflow limitation. (1-4) Depending on the population studied, between 20% and 70% of patients with COPD are underweight. (1,5-9) Significant weight loss, resulting in a body weight of < 90% of ideal body weight (IBW), occurs in 25% (1,9) to 43% (6) of patients with COPD. It has been estimated that 14% of COPD patients lose > 50% of their premorbid weight. (2) In the United States alone, approximately 16 million people have COPD. (10) Therefore, at least 4 million patients have experienced significant unintentional weight loss. The prevalence can be expected to increase as the smoking population ages.
Weight loss and the attendant loss of lean body mass (LBM) have been associated with skeletal muscle dysfunction, impacting not only the respiratory musculature, but also having effects on peripheral skeletal muscle function. (11) This results in weakness and fatigue, contributing not only to dyspnea, but also leading to decreased strength, impaired mobility, and an increased risk for falling. Overall, quality of life (QOL) is adversely affected. (11,12) Significant weight loss has been found to begin, on average, 3.5 years before death. (2) Following the onset of unintentional weight loss, mortality reaches 30% in 3 years, and climbs to 50% in 5 years. (1,2) Exercise ability is influenced by both airflow limitation and body weight. As reported from the Intermittent Positive Pressure Breathing trial, (1) the level of exercise achieved declines with decreasing body weight. Even when the FE[V.sub.1] is < 35% predicted, denoting severe airflow impairment, the level of exercise performed is 50% greater in those with COPD who are above IBW than by those who are significantly underweight (< 90% IBW). (1) Body weight correlated with exercise capacity (p < 0.0001).
Reversal of weight loss has been associated with improved outcomes, including increased muscle strength and exercise capacity, as well as increased survival. (13-15) Schols and colleagues (15) demonstrated that, while COPD-associated weight loss and decreased body mass index (BMI) are associated with increased mortality, weight gain in this population is associated with increased survival. Additionally, Rogers et al (14) used dietary supplementation to achieve weight gain in patients with COPD and weight loss, and this was associated with improvements in 12-min walking distance, handgrip strength, and maximum inspiratory pressure (PImax), and maximum expiratory pressure (PEmax). The control group, which did not receive dietary supplementation, experienced a variable response pattern for these parameters. Similarly, Efthimiou et al (13) also demonstrated an association between increasing weight using dietary supplementation, and increased respiratory and skeletal muscle strength, with subsequent declines in each parameter when supplementation was discontinued and weight was again lost.
Restoration of body weight may be difficult to achieve and to maintain using nutritional intervention alone. (14,16) Furthermore, increased caloric intake may promote a disproportionate amount of fat accrual. (16) It is questionable whether such an increase in adipose tissue confers advantages to the patient with COPD. (16)
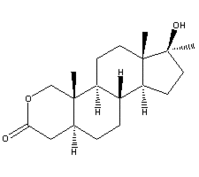
Oxandrolone is an oral anabolic steroid approved as an adjunct to help restore weight to patients who have lost weight due to chronic infection, severe trauma, extensive surgery, or who fail to gain or maintain weight due to unknown pathophysiologic reasons, and to help offset the catabolism associated with the long-term use of corticosteroids. (17) Oxandrolone has high anabolic activity, approximately 6.3 times that of methyltestosterone, (18) yet a low potential for androgenic effects. (19-21) While the ratio of anabolic to androgenic potency with testosterone, for example, is 1:1, the ratio of these effects with oxandrolone is between 3:1 and 13:1. (22) A good safety profile has been established for oxandrolone in > 30 years of clinical use. (20-21) Therefore, oxandrolone was evaluated as an adjunct to restoring weight in patients with COPD-associated weight loss.
MATERIALS AND METHODS
This study was a community based, multicenter, prospective clinical trial conducted at 25 sites in the United States between February 1998 and February 2000. Patients meeting the eligibility criteria were provided with oxandrolone, 10 mg bid, orally administered for 4 months with regularly scheduled interim evaluations. Institutional Review Board approval and ongoing oversight of the study was provided. All patients were > 40 years old and had a history of involuntary weight loss resulting in a body weight [less than or equal to] 90% of their IBW, a Karnofsky performance status score [greater than or equal to] 50, and a stable medication regimen for 1 month prior to beginning oxandrolone therapy. All patients enrolled were in stable cardiac and pulmonary condition. Patients having received any anabolic agent in the past 6 months or any investigational treatment within the past 30 days were excluded. Also excluded were any men with a prostate-specific antigen level greater than normal for age, or patients with clinically significant hepatic or renal disease, or hypercalcemia. COPD diagnosis was confirmed by a decreased FE[V.sub.1] < 50% of predicted, and FE[V.sub.1]/FVC ratio < 0.7. Written informed consent was obtained from each patient. Patients were advised to be as physically active as tolerated, and to ingest a diet sufficient in calories and protein. However, no specific nutritional or exercise program was provided. Assessments were made at baseline, and on completion of month 2 and month 4.
Spirometry was conducted in accordance with American Thoracic Society guidelines, (23) and included FE[V.sub.1], FE[V.sub.1]/FVC ratio, PImax, PEmax, and maximum voluntary ventilation projected to 1 min. Body composition was determined by bioelectric impedance analysis (Quantum Model 101-Q; RJL Systems; Clinton Township, MI). (24) Body weight was obtained using a calibrated balance or electronic scale in a consistent fashion (light clothing, no shoes) with repeated measures to ensure consistency. Karnofsky performance status, a measure of functional ability, was assessed by the investigator at each visit based on observation and subjective feedback from the patient. A score of 50 indicates the patient requires considerable assistance or medical care. Six-minute walking distance (6MWD) was performed indoors along a measured hallway with comfortable ambient temperature, with recording of the total distance covered by the patient during that time. Each patient set his or her own pace and could rest as needed. An 11-question survey with a 100-mm visual analog scale (VAS) was created for this study and was completed by each patient at each visit. Questions pertained to aspects of dyspnea, energy and fatigue, sleep quality, and appetite. Scores were marked by the patient anywhere from 0 to 100 mm along the continuous scale. The better the patient feels or functions, the higher the score. Scores were measured and compared between visits. This instrument was not previously validated.
This pilot study was not powered for analysis of a specific parameter. All patients' data were employed in an intent-to-treat fashion. For the efficacy analysis, the one-sample Student t test was used to compare mean changes from baseline in body weight, BMI, and body composition at each interim evaluation at p = 0.05 level. The one-sample Student t test was also used to compare increases in respiratory muscle strength, endurance and overall functional capacity, and changes in QOL measures. All p values < 0.05 were considered statistically significant.
RESULTS
One hundred twenty-eight patients met the selection criteria and were enrolled in the study. Demographics are shown in Table 1. There were 71 women and 57 men (n = 128). Mean age was 68.8 years. Mean [+ or -] SD percentage of IBW was 79 [+ or -] 9.2%. Mean FE[V.sub.1] was 34 [+ or -] 15.83% of predicted, and mean FE[V.sub.1]/FVC ratio was 0.47 [+ or -] 0.34. Mean BMI was 17.8 [+ or -] 2.15. Disposition of the patients over the course of the study is shown in Table 2. Although 49 patients received oxandrolone for the entire 4 months, 55 patients are included in the analyses, as data for 6 patients who discontinued study between the month 2 and month 4 visits were captured and carried forward.
Assessments were made at baseline and at month 2 and month 4. These included body weight, body composition, review of concomitant medications, assessment for adverse events, and laboratory analysis for safety surveillance (CBC, chemistry panel with liver function tests, total cholesterol, and triglycerides). Prostate-specific antigen and testosterone were measured at baseline.
At month 2, 72 of 82 patients (88%) had gained a mean of 6.0 [+ or -] 4.36 lb (p < 0.05). Ten of 82 patients (12%) had a mean weight loss of 1.7 [+ or -] 2.15 lb (not statistically significant [NS]). For all patients, there was a mean weight gain of 5.1 [+ or -] 4.86 lb (p < 0.05). By month 4, 46 of 55 patients (84%) gained a mean of 6.0 [+ or -] 5.83 lb (p < 0.05). Nine of 55 patients (16%) had a mean weight loss of 1.8 [+ or -] 1.74 lb (p = NS). For all patients, there was a mean gain of 4.7 [+ or -] 6.09 lb (p < 0.05) at 4 months (Fig 1). Body composition, as determined by bioelectric impedance analysis, showed a mean increase in body cell mass (BCM) of 3.0 [+ or -] 2.6 lb (p < 0.05) and a mean increase in fat mass of 1.2 [+ or -] 4.6 lb (NS) [Fig 2]. Changes in weight and body composition were comparable between genders. Baseline serum testosterone levels were available for 30 men. Twelve men were hypogonadal (total testosterone level < 280 ng/dL) at baseline. Their mean weight gain was 4.48 [+ or -] 3.83 lb at month 2 (n = 12) and 1.35 [+ or -] 4.88 lb at month 4 (n = 9). The eugonadal men (testosterone level > 280 ng/dL) gained a mean of 5.34 [+ or -] 4.72 lb at month 2 (n=17) and 6.20 [+ or -] 5.02 lb at month 4 (n = 12). Body composition analysis at month 4 revealed that BCM increased a mean of 6.25 [+ or -] 0.92 lb and fat mass increased 2.07 [+ or -] 6.27 lb for the hypogonadal men (n = 3). The eugonadal men (n = 10) gained 3.17 [+ or -] 2.68 lb of BCM and 1.63 [+ or -] 3.39 lb of fat mass.
[FIGURE 2 OMITTED]
As shown in Table 3, spirometry findings did not change significantly. Patients completing the study had a mean increase in 6MWD of 11 [+ or -] 77 m (NS) compared to baseline (n = 44). However, 23 of the 42 patients (55%) for whom there are 6MWD data demonstrated increased 6MWD, with a mean increase of 65 [+ or -] 57 m (NS). Total VAS scores (sum of all parameters) were unchanged at both month 2 and month 4. However, mean appetite scores increased 1.28 [+ or -] 2.85 cm (p [less than or equal to] 0.01) and 0.75 [+ or -] 3.0 cm (p = 0.07) at month 2 and month 4, respectively.
Karnofsky performance status remained stable for the majority of patients (62% at month 2 and 53% at month 4) and was similar between men and women. However, Karnofsky performance status scores improved for 26% of patients by month 2 (p = 0.02) and for 33% of patients by month 4 (p = 0.02). Mean baseline Karnofsky performance status score was 70.1 [+ or -] 11.1, and increased to a mean of 73.3 [+ or -] 12.9 at month 2 (p = 0.02) and to 74.0 [+ or -] 12.7 at month 4 (p = 0.02; Table 4). Changes were again comparable between genders. Karnofsky performance status of older patients ([greater than or equal to] 65 years) did not change significantly, with mean Karnofsky performance status scores of 71.2 [+ or -] 11.2 at baseline (n = 41) and 70.4 [+ or -] 12.7 at month 4 (n = 25) [NS]. The younger patients (< 65 years) had greater mean increase in Karnofsky performance status, increasing from 71.3 [+ or -] 11.8 (n=31) to 77.6 [+ or -] 12.6 (n=21) [p = 0.02], although baseline status for the two age groups was similar.
Changes in mean laboratory values were analyzed. For all patients, mean changes from baseline to month 2 and month 4 in alkaline phosphatase, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total cholesterol were each statistically significant. Mean changes in these parameters in the older subset ([greater than or equal to] 65 years old) were each significant, as were mean changes in patients < 65 years old for alkaline phosphatase, ALT, and cholesterol. The mean change in AST for the younger subgroup was NS at month 2, but was significant at month 4. Between age groups, however, the only statistically significant laboratory changes were AST and cholesterol. There were no significant changes from baseline in mean BUN. The change in mean creatinine level (0.08 [+ or -] 0.13 mg/dL) was significant only for those < 65 years old at month 4. Use of respiratory-specific medications declined at successive visits, as shown in Table 5.
Elderly Subset
A post hoc analysis of data from patients [greater than or equal to] 65 years old was performed to determine if there are age-related differences in the effects of oxandrolone. Sixty-two percent of enrolled patients were > 65 years old (n = 79). The mean age of this subset was 75 years. Baseline spirometry, 6MWD, and patient questionnaire for the elderly were congruent with those of the study group as a whole. Twenty-five of the 32 elderly patients (78%) completing the study gained a mean of 5.3 [+ or -] 4.9 lb (p < 0.05). Seven patients (22%) lost a mean of 1.69 [+ or -] 1.92 lb (NS). BCM (n = 29) increased 2.8 [+ or -] 2.8 lb (p < 0.05), while fat mass (n = 29) increased 0.92 [+ or -] 4.2 lb (NS). Eleven of the 26 elderly patients (42%) for whom there were 6MWD data demonstrated an increase in 6MWD, a mean increase of 69.5 [+ or -] 72 m (NS). Total VAS scores were unchanged, but the mean appetite scores increased 1.41 [+ or -] 2.8 cm (p<0.01) and 0.65 [+ or -] 3.3 cm (NS) at month 2 (n = 44) and month 4 (n = 32), respectively. Karnofsky performance status score did not change; mean score was 71 at baseline and at month 4.
Adverse Experiences
Adverse experiences deemed by the investigator to be possibly or probably related to oxandrolone involved 49 patients (38%) and are delineated in Table 6. Edema occurred or was exacerbated in 22 of the 128 patients (17.2%). GI disorders were noted in 12 of 128 patients (9.4%), with nausea being the most frequent complaint. Respiratory disorders affected 12 of 128 patients (9.4%). Miscellaneous skin disorders (including alopecia, see below) occurred in 11 of 128 patients (8.6%), and musculoskeletal complaints were observed in 8 of 128 patients (6.3%). Seven of 128 patients (5.5%) complained of fatigue. Ten of 87 patients (11.5%) for whom there were postbaseline laboratory assessments had elevations of hepatic transaminases > 2.5 times the upper normal limit. None of the patients were symptomatic. Of these patients, 5 of 87 patients (5.7%) had elevations more than five times the upper normal limit (World Health Organization grade III moderate adverse event criteria). Six of the 10 patients (6.9% of the 87 patients) were discontinued from the study prior to month 4. A total of 23 of 128 patients (18%) discontinued in association with conditions attributed to oxandrolone, including one death.
Six women (11.8% of the women) had androgenic side effects and were withdrawn from study. Four women complained of alopecia, one woman had cliteromegaly develop, and another woman had hirsutism and deepening of her voice develop. The symptoms in the latter patient progressively diminished with time following drug cessation. Follow-up status of the other women is not available.
A post hoc analysis was conducted to determine if baseline factors could be identified that might predict patients who would respond to oxandrolone, to have adverse events, or discontinue early. Increased age and low QOL score each correlated with early discontinuation. Patients with adverse experiences, whether related to the drug or not, were more likely to discontinue than patients without such events. Patients with adverse events related to oxandrolone were significantly more likely to discontinue than to remain on study. No predictors of adverse experiences were identified. With regard to predictors of response, a gender effect is observed. Specifically, women had a greater gain as a percentage of their baseline body weight, and a greater absolute weight gain. However, there was no significant difference between genders in BCM gain. Rather, the women gained slightly more fat mass (difference is NS). This difference in response may be because androgens are relatively more novel to women than to men.
DISCUSSION
This is the first clinical trial evaluating the effects of oxandrolone in patients with COPD-associated involuntary weight loss. This study demonstrates that the addition of oxandrolone to the treatment of patients with COPD and weight loss increases body weight, primarily in the form of LBM. This effect is significant by 2 months and is maintained through the 4 months of treatment, despite the absence of deliberate dietary or exercise regimens. However, the maintenance of weight following cessation of oxandrolone is not known, as this was not assessed.
The literature has dearly established that involuntary weight loss occurs commonly in patients with COPD, and that weight loss is a predictor of mortality independent of the severity of COPD. (1-4,9,14,24,25) It has also been demonstrated that weight loss in COPD patients is associated with increased mortality, impairment of muscle function, and reduced QOL. (11-13) The restoration of weight in patients with COPD is associated with increased survival, increased strength and exercise capacity, and improved QOL. (13-15) Schols and colleagues (15) determined that weight restoration > 2 kg over 8 weeks was a significant predictor of survival. The present study demonstrates that this amount of weight gain can be achieved with oxandrolone therapy. Improvements in pulmonary function, although not seen in this trial, have also been observed with weight restoration. (13,14) Yet, the restoration of weight in this population has remained difficult to achieve by nutritional intervention alone. While progestational agents and appetite stimulants are sometimes employed in an endeavor to increase weight, such weight gain is primarily in the form of adipose tissue, (16,26,27) and it is of questionable benefit to the patient. (28) The parenteral anabolic-androgenic steroid, nandrolone decanoate, has been shown to promote weight and strength in similar COPD patients within the context of an inpatient rehabilitation setting. (16) However, nandrolone requires parenteral administration, does not have a high separation of anabolic and androgenic effects, (22) and is not approved for weight restoration.
In the current study, use of oxandrolone was associated with increased 6MWD of > 65 m in the majority of patients. Efthimiou et al, (13) as well as Redelmeier et al, (29) have demonstrated that an increase in 6MWD of [greater than or equal to] 53 m correlates with subjective improvement in the clinical condition of patients with COPD when validated questionnaires are utilized. (13,29) This suggests an improvement in health status may have occurred in the current study that was not detected by the simple VAS employed. However, this benefit may be inferred from the significant improvement in the Karnofsky performance status scores.
We found that the use of respiratory medications progressively declined during the study, although there was no corresponding improvement observed in pulmonary function tests. The significance of this finding is not clear. However, the study was not powered to detect changes in these parameters. Patients whose pulmonary status worsened and resulted in early discontinuation from the study were likely to have continued or increased their use of respiratory medications. The continuing patients may, therefore, have been less symptomatic from their COPD. Nonetheless, the association of oxandrolone with reduction in the use of respiratory medications by those remaining in the study is an intriguing observation.
Certainly, there are limitations inherent in an open-label study absent a control group. Additionally, this study was impacted by a high rate of early discontinuations, and this population was prone to adverse experiences. Patients with adverse experiences, related to drug or not, were more likely to discontinue than those without an adverse event. However, 56 of the 79 dropouts (71%) were for personal reasons or due to events unrelated to drug. Disease exacerbation is a common occurrence in the patient with moderate-to-severe COPD, with two to three exacerbations per year. (30-32) Therefore, it is not unexpected that a large proportion of patients who were free of exacerbation for at least i month prior to study would have an exacerbation during the ensuing 4-month study period. It is, therefore, impossible to determine a relationship to study drug without a comparable control group. In some cases, patients elected to discontinue study at the time of COPD exacerbation or other intercurrent illness, although they were otherwise eligible to continue on the study. Patients with moderate-to-severe COPD are known to have high levels of anxiety and depression, and this no doubt influenced that decision. (33) A post hoc analysis found that increased age and low QOL score each correlated with early discontinuation. The low QOL score may reflect depression, and this has been reported in patients with COPD using validated tools. (33) No other predictors of dropout were revealed.
While 38% of study patients had at least one adverse event attributed to the study drug (Table 6), only 18% discontinued for this reason. Despite the physicians' assessment as "possibly related" to oxandrolone, a large number of the listed events could conceivably be due to underlying disease or intercurrent illness. Specifically, many of the respiratory, GI and skin disorders, and musculoskeletal complaints, may not actually be drug related judging by previous oxandrolone experience.
Edema is a known complication of this class of drugs, associated with sodium and fluid retention. Edema was reported in 22 of the 128 patients (17.2%), most of whom had a history of edema. This was readily managed by dose reduction or cessation, and by the use of diuretics. Careful patient selection to screen for a history of edema and appropriate monitoring during administration may minimize this occurrence. Nausea was the predominant GI symptom, and is another known potential adverse experience with oxandrolone. This may be minimized by taking oxandrolone with meals, or by further dividing the daily dose.
Changes in a number of laboratory values were statistically significant, yet the majority of these shifts were either within the normal ranges or less than two times normal, and were not clinically significant. Although hepatic abnormalities are known to occur with the anabolic-androgenic steroid class, the occurrence with oxandrolone appears to be relatively benign. Elevations of transaminases, with ALT higher than AST, were also observed in this trial with oxandrolone, prompting discontinuation of oxandrolone in 6.9% of patients. However, all patients with elevated transaminases were generally well subjectively, and abnormalities were limited to the transaminases with neither clinical nor laboratory evidence of cholestasis (bilirubin or alkaline phosphatase). Furthermore, this was a transient transaminitis, in some cases resolving while still receiving the study drug, and in all cases returning to the normal range within 3 to 6 weeks of drug cessation without sequelae. Drug-induced dyslipidemia is another class-related effect also observed with oxandrolone. In this study, both total cholesterol and triglyceride levels tended to decrease from baseline. However, lipid alterations of such short duration as in this trial are not known to affect the risk of atherosclerotic disease.
Another noteworthy observation in this study was that oxandrolone was very well-tolerated by women, who comprised more than half the study sample. We believe this is the first study of anabolic steroids in patients with COPD associated weight loss in which women are significantly represented. Although treated with the maximum approved daily dose for adults, only 6 of 71 women (11.8%) had androgenic effects. This is important, as the prevalence of women with symptomatic COPD and associated weight loss is expected to rise as a result of the increased numbers of women smokers over the past few decades.
Although not presented herewith, proinflammatory cytokines and leptin levels were evaluated on the patients studied at one center. These were elevated at baseline and were observed to have decreased by the end of treatment, in association with increased body weight. (34) These results underscore the systemic nature of COPD and associated weight loss, and the potential role for oxandrolone in the management of this problem. The results of an ongoing, randomized, placebo-controlled COPD trial are anticipated to provide more definitive information regarding the effects of oxandrolone in this population.
CONCLUSION
Oxandrolone is effective as an adjunct in restoring weight for patients with COPD-associated involuntary weight loss, including women and the elderly. Weight gain is primarily in the form of LBM. For the majority of patients who had an increase in 6MWD following 4 months of oxandrolone therapy, the increase in walking distance was clinically significant, and this is supported by the increase in Karnofsky performance status scores. Oxandrolone was relatively well tolerated and, therefore, should be a consideration in the comprehensive care of patients with COPD and weight loss.
APPENDIX
Participating investigators: are Robert J. Albin, MD (Atlanta, GA); Lynn Anderson, MD (Bay Pines, FL); Robert Bowen, MD (Martinsburg, WV); Jeffrey Carr, MD (Lake Mary, FL); Henry D. Covelli, MD (Coeur D'Alene, ID); Ronald Creasman, MD (Mesa, AZ); Harold Fleming, MD (Spartanburg, SC); Jonathan Flescher, MD (Raleigh, NC); Glen Giessel, MD (Richmond, VA); Mark Gotfried, MD (Phoenix, AZ); David Hagaman, MD (Nashville, TN); Ali Imam, MD (Corona, CA); William Jannetti, MD (Buena Park, CA); Eric Jimenez, MD (Danbury, CT); Robert Lapidus, MD (Wheat Ridge, CO); M. Douglas Lee, MD (Wilmington, NC); Roy Levinson, MD (Willingboro, NJ); Bernard A. Michlin, MD (San Diego, CA); James O'Halloran, DO (Torrington, CT); Lawrence Repsher, MD (Wheat Ridge, CO); Steve Sahn, MD (Charleston, SC); Geoffrey Serfilippi, MD (Albany, NY); Stewart Simon, MD (Austell, GA); William Sokol, MD (Newport Beach, CA); Bradley Spitz, MD (San Diego, CA); James R. Taylor, MD (Tacoma, WA); Manuel S. Villareal, MD (Cincinnati, OH); Shing-Shing Yeh, MD, PhD (Northport, NY); Richard Zuwallack, MD (Hartford, CT).
REFERENCES
(1) Wilson DO, Rogers RM, Wright EC, et al. Body weight in chronic obstructive pulmonary disease: the National Institutes of Health Intermittent Positive-pressure Breathing Trial. Am Rev Respir Dis 1989; 139:1435-1438
(2) Vandenbergh E, Van de Woestijne KP, Gyselen A. Weight changes in the terminal stages of chronic obstructive pulmonary disease. Am Rev Respir Dis 1967:556-566
(3) Gray-Donald K, Gibbons L, Shapiro SH, et al. Nutritional status and mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996; 153:961-966
(4) Laaban JP, Kouchakju B, Dore MF, et al. Nutritional status of patients with chronic obstructive pulmonary disease and acute respiratory failure. Chest 1993; 103:1362-1368
(5) Mitchell RS. Chronic obstructive bronchopulmonary disease: I; clinical features. Am Rev Respir Dis 1964; 89:360-371
(6) Openbrier D, Irwin M, Rogers RM. Nutritional status and lung function in patients with emphysema and chronic bronchitis. Chest 1983; 83:17-22
(7) Braun SR, Keim NL, Dixon RM, et al. The prevalence and determinants of nutritional changes in chronic obstructive pulmonary disease. Chest 1984; 86:558-563
(8) Fiaccadori E, DelCanale S, Coffrini E, et al. Hypercapnic-hypoxemic chronic obstructive pulmonary disease (COPD): influence of severity of COPD on nutritional status. Am J Clin Nutr 1988; 48:680-685
(9) Schols AMWJ, Soeters PB, Dingemans AMC, et al. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis 1993; 147:1151-1156
(10) Murray JF, Dadel JA, eds. Textbook of respiratory medicine. 2nd ed. Philadelphia, PA: WB Saunders, 1994; 1331-1397
(11) Rochester DF. Malnutrition and the respiratory muscles. Clin Chest Med 1986; 7:91-99
(12) Ketelaars CAJ, Schlosser MAG, Mosert R. Determinants of health-related quality of life in patients with chronic obstructive pulmonary disease. Thorax 1996; 51:39-43
(13) Efthimiou J, Fleming J, Gomes C, et al. The effect of supplementary oral nutrition in poorly nourished patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1988; 137:1075-1082
(14) Rogers RM, Donahoe M, Costantino J. Physiologic effects of oral supplemental feeding in malnourished patients with chronic obstructive pulmonary disease: a randomized control study. Am Rev Respir Dis 1992; 146:1511-1517
(15) Schols AMWJ, Slangen J, Volovies L, et al. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157:1791-1797
(16) Schols AMWJ, Soeters PB, Mostert R, et al. Physiologic effects of nutritional support and anabolic steroids in patients with chronic obstructive pulmonary disease: a placebo-controlled randomized trial. Am J Respir Crit Care Med 1995; 152:1268-1274
(17) Oxandrin package insert. Iselin, NJ: BTG Pharmaceuticals, 1998
(18) Fox M, Minor AS, Liddle GW. Oxandrolone: a potent anabolic steroid of novel chemical configuration. J Clin Endocrinol Metab 1962; 22:921-924
(19) Bonkovsky HL, Fiellin DA, Smith GS, et al, A randomized, controlled trial of treatment of alcoholic hepatitis with parental nutrition and oxandrolone: I; short-term effects on liver function. Am J Gastroenterol 1991; 86:1200-1208
(20) Blizzard RM, HindMarsh PC, Stanhope R. Oxandrolone therapy: 25 years experience. Growth Genet Horm 1991; 7:1-15
(21) Gherondache CN, Dowling WJ, Pincus SD. Metabolic changes induced in elderly patients with an anabolic steroid (oxandrolone). J Gerontol 1967:290-300
(22) Meyers FH, Jawetz E, Goldfien A. The gonadal hormones and inhibitors. In: Review of medical pharmacology. Los Altos, CA: Lange Medical Publications, 1972; 411-415
(23) Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease: American Thoracic Society. Am J Respir Grit Care Med 1995; 152(5 Pt 1):S77-S121
(24) Schols AM, Wouters, EMF, Soeters PB. Body composition with bioelectric impedance analysis compared with deuterium dilution and skinfold anthropometry in patients with chronic obstructive pulmonary disease. Am J Clin Nutr 1991; 53:421-424
(25) Wouters EFM, Schols AMWJ. Prevalence and pathophysiology of nutritional depletion in chronic obstructive pulmonary disease. Respir Med 1993; 87:45-47
(26) Loprizini CL, Schaid DJ, Dose AM, et al. Body-composition changes in patients that gain weight while receiving megesterol acetate. J Clin Oncol 1993; 11:152-154
(27) Congleton J. The pulmonary cachexia syndrome: aspects of energy balance. Proc Nutr Soc 1999; 58:321-328
(28) Langer CJ, Hoffman JP, Ottery FD. Clinical significance of weight loss in cancer patients: rationale for the use of anabolic agents in the treatment of cancer-related cachexia. Nutrition 2001; 17:S1-S22
(29) Redelmeier DA, Bayoumi AM, Goldstein RS, et al. Interpreting small differences in functional status: the six minute walk test in chronic lung disease patients Am J Respir Crit Care Med 1997; 155:1278-1282
(30) Anthonisen NR, Manfreda J, Warren CP. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987; 106:196-204
(31) Seemungal TA, Donaldson GC, Paul EA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157(5 pt 1):1418-1422
(32) Seemungal TA, Donaldson GC, Bhowmik A. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 161: 1608-1613
(33) Okubadejo AA, Jones PW, Wedzicha JA. Quality of life in patients with chronic obstructive pulmonary disease and severe hypoxaemia. Thorax 1996; 51:44-47
(34) Yeh S. Relationship between body composition and cytokines in cachectic COPD patients. Presented at: Annual Meeting of the Endocrine Society, June 20-28, 2001, Denver, CO
Shing-shing Yeh, MD; Bernadette DeGuzman, BA; and Ted Kramer, MD; for the M012 Study Group ([dagger])
* From the Veterans' Affairs Medical Center (Dr. Yeh), Northport, NY; and Bio-Technology General Corporation (Ms. DeGuzman and Dr. Kramer), Iselin, NJ.
([dagger]) A list of study participants is given in the Appendix. Dr. Kramer and Ms. DeGuzman are employees of BioTechnology General.
Study support provided by Bio-Technology General Corporation.
Manuscript received August 10, 2001; revision accepted February 5, 2002.
Correspondence to: Ted Kramer, MD, Director, Medical Affairs, Bio-Technology General Corporation, 70 Wood Ave South, Iselin, NJ 08830; e-mail: tkramer@btgc.com
COPYRIGHT 2002 American College of Chest Physicians
COPYRIGHT 2002 Gale Group