Business Editors/Medical Writers
PITTSBURGH--(BUSINESS WIRE)--Aug. 21, 2001
Mylan Laboratories Inc. (NYSE:MYL) announced today that the Food and Drug Administration has approved its Abbreviated New Drug Applications (ANDAs) for Oxaprozin Tablets, 600 mg and Spironolactone Tablets USP, 25 mg, 50 mg and 100 mg.
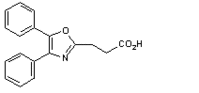
Oxaprozin is the generic equivalent of G.D. Searle and Company's Daypro(R) and is a nonsteroidal anti-inflammatory (NSAID).
Spironolactone is the generic equivalent of Searle Pharmaceuticals, Inc.'s Aldactone(R) indicated for essential hypertension, primary hyperaldosteronism, edematous conditions for patients and hypokalemia.
Both products will be manufactured in Mylan's Morgantown, West Virginia facility and shipping will begin in the near future.
Mylan Laboratories Inc. is a leading pharmaceutical company that develops, manufactures and markets generic and proprietary prescription pharmaceutical products. The Company markets an extensive line of generic products through three business units, Mylan Pharmaceuticals Inc., Mylan Technologies Inc., and UDL Laboratories, Inc. and branded products through its Bertek Pharmaceuticals Inc. subsidiary. These products are manufactured and sold in a variety of dosage forms including immediate and extended-release oral tablets and capsules and transdermal patches as well as repackaging and marketing multi-source and single-source products in unit dose form. For more information, visit www.mylan.com.
COPYRIGHT 2001 Business Wire
COPYRIGHT 2001 Gale Group