Business/Health Editors
NEW YORK--(BW HealthWire)--Feb. 1, 2001
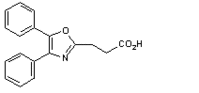
Eon Labs Manufacturing, Inc. announced today that they have received FDA approval for Oxaprozin 600mg Tablets, the first generic for the arthritis medication marketed under the brand name, Daypro(R)(1). Sales of Daypro(R) exceeded $160 million in 2000. Eon Oxaprozin Tablets are available in 100 and 500 count bottles.
"Oxaprozin is the first approval from Eon Labs in 2001", said Frank Della Fera, R.Ph., Vice President of Sales and Marketing for Eon Labs. "Our first approval of the year is also our initial "first to market" product of the new year", added Della Fera. "We are extremely optimistic about the prospects for 2001, and believe that we will better the 14 approvals that Eon received last year", he concluded.
Eon Labs Manufacturing, Inc. is one of the leading generic pharmaceutical companies in the U.S. For further information on Oxaprozin Tablets or other products manufactured and distributed by Eon Labs, please call 1-800-526-0225, or write to Eon Labs Manufacturing, Inc., 227-15 N. Conduit Avenue, Laurelton, N.Y. 11413.
Oxaprozin 600mg Tablets bottle photo available upon request.
(1) Daypro is a registered trademark of G.D. Searle & Co., and is not
affiliated with Eon Labs Manufacturing, Inc.
COPYRIGHT 2001 Business Wire
COPYRIGHT 2001 Gale Group