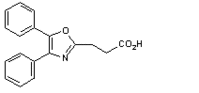
The Food and Drug Administration recently approved three abbreviated new drug applications from Watson: oxaprozin, methylphenidate hydrochloride and microgestin. Oxaprozin is the equivalent to G.D. Searle and Co.'s Daypro for osteoarthritis and rheumatoid arthritis. Methylphenidate hydrochloride is the generic equivalent to Novartis Pharmaceuticals' Ritalin for children with serious behavioral problems. Microgestin tablets are the therapeutic equivalent to Parke Davis'/Pfizer's Loestrin, an oral contraceptive. Additionally, Watson and partner Genelabs Technologies are waiting for FDA review of Aslera (prasterone), an investigational lupus drug. The review is scheduled for April 19. If approved, Watson plans to launch the drug in summer 2001.
COPYRIGHT 2001 Lebhar-Friedman, Inc.
COPYRIGHT 2001 Gale Group