Because many antihypertensive drugs can affect airway function, the treatment of hypertension in patients with airway dysfunction is complex. For example, the worsening or precipitation of asthma by [beta]-adrenoceptor antagonists is well-recognized, but [[beta].sub.1]-adrenoceptor blockers that exert mild [[beta].sub.2]-agonist effects, and those that modulate the endogenous production of nitric oxide, affect airway function to a lesser extent. Therapy with selective [[alpha].sub.1]-blockers is not contraindicated in cases of chronic airway obstruction. Conversely, [[alpha].sub.2]-agonists must not be given to asthmatic subjects because they can adversely affect the bronchi. Calcium channel blockers do not exert severe side effects on the airways. Angiotensin-converting enzyme inhibitors may cause cough and exacerbate or even induce asthma; however, angiotensin II type I (A[T.sub.1]) antagonists do not cause cough. 5-Hydroxytryptamine modifiers such as urapidil are a treatment option for patients with chronic airway obstruction. In patients with airway dysfunction, we suggest treatment with thiazide diuretics as the initial drug choice, and calcium channel blockers if the response is poor. In the case of no response, calcium channel blockers alone must be used. However, there is no strict rule because individual patients may respond differently to individual drugs and drug combinations. Consequently, it is important to adopt a flexible approach. For patients who are unresponsive to the aforementioned drugs, A[T.sub.1] receptor antagonists, newer [[beta].sub.1]-adrenoceptor-blocking agents with ancillary properties (eg, celiprolol or nebivolol), and/or vasodilators can be considered. (CHEST 2002; 121:230-241)
Key words: airway response; antihypertensive drums; arterial hypertension; asthma; COPD
Abbreviations: A[T.sub.1] = angiotensin II type 1; ACE = angiotensin-converting enzyme; 5-HT = 5-hydroxytryptamine; ISA = intrinsic sympathomimetic activity; P[C.sub.20] = provocative concentration of methacholine causing a 20% fall in FE[V.sub.1]; PEFR = peak expiratory flow rate; PG = prostaglandin
**********
The primary aim of the pharmacologic treatment of hypertension is to achieve optimal or normal BP in order to reduce the risk of cardiovascular morbidity and mortality. According to the results of the Hypertension Optimal Treatment trial, (1) the acceptable BP level is < 140/90 mm Hg, and the optimal BP should be < 130/<85 mm Hg. However, patients whose BP was < 150/90 mm Hg were apparently not disadvantaged.
Achieving target BP levels depends on a number of factors, among which are the patient's risk factors. Thus, attempts should be made to eliminate all reversible risk factors (eg, smoking, elevated cholesterol, and diabetes). Associated clinical conditions should be managed, and elevated BP levels should be treated. Drug treatment should be adjunctive to appropriate lifestyle changes (eg, dietary changes, smoking cessation, and regular exercise). (2)
The Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (2) recommends a diuretic or a [beta]-blocker (Table 1) for initial treatment when hypertension is uncomplicated and there are no contraindications to these medications. Nonetheless, according to the World Health Organization-International Society of Hypertension report, (3) any of the available medications (ie, diuretics, [beta]-blockers, angiotensin-converting enzyme [ACE] inhibitors, angiotensin II type 1 [A[T.sub.1]] receptor blockers, and, in some instances, [alpha]-blockers) are acceptable for initial therapy.
The concomitant presence of arterial hypertension and chronic airway obstruction (ie, asthma or COPD) is not unusual. In fact, hypertension is the most common comorbid disease in COPD patients. Antonelli Incalzi et al (4) reported that 28% of 270 patients consecutively discharged from a university hospital after an acute exacerbation of COPD experienced hypertension. Deplorably, many antihypertensive medications recommended by the Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure and by the World Health Organization-International Society of Hypertension report might have deleterious effects on airways (Fig 1). (5)
[FIGURE 1 OMITTED]
We have reviewed how antihypertensive drugs can affect airway function. This body of data can help physicians to select treatment for patients with hypertension who also have chronic airway obstruction.
[beta]-ADRENERGIC ANTAGONISTS
[beta]-Adrenoceptors are widely distributed in human airways and alveoli. (6) [[beta].sub.2]-Receptors are the predominant adrenoceptors on airway smooth muscle, whereas [[beta].sub.1]-receptors account for 10% and 30% of the adrenoceptors on submucosal glands and alveolar walls, respectively. Being competitive inhibitors of [beta]-adrenoceptors, [beta]-adrenoceptor-blocking drugs can cause bronchoconstriction. The severity of the bronchoconstrictor response to a given [beta]-adrenoceptor-blocking agent is not predictable, (7) and occurs mainly in patients with reversible bronchial obstruction. (8) These blocking agents can cause severe bronchoconstriction even in subjects with mild asthma. The dose of [beta]-adrenoceptor-blocking drugs administered may be low, as in the case of timolol, a nonselective [beta]-adrenoceptor blocker used to treat glaucoma. (9)
Nonselective [beta]-Adrenoceptor-Blocking Drugs
Nonselective [beta] ([[beta].sub.1] + [[beta].sub.2])-adrenoceptor-blocking agents can precipitate bronchospasm in patients with asthma. Propranolol and other nonselective [beta]-adrenergic antagonists, such as timolol and nadolol, block [[beta].sub.2]-adrenoceptors in bronchial smooth muscle. (10) This pharmacologic action usually has little effect on pulmonary function in healthy individuals. However, the antagonistic effect is 66-fold greater in symptomatic asthma patients than in nonasthmatic subjects with inhaled propranolol, and sixfold greater with IV propranolol, (11) Consequently, in patients with asthma or COPD, this treatment can lead to life-threatening bronchoconstriction. (12)
[[beta].sub.1] - Selective Adrenoceptor-Blocking Drugs
Most of the adverse pulmonary effects exerted by [beta]-adrenoceptor-blocking drugs in asthma or COPD patients are related to interference with [[beta].sub.2]-adrenoceptor-mediated bronchodilation. Various studies (13-18) have identified relevant differences in the impact on airway function, depending on whether a [beta]-blocker is [[beta].sub.1]-selective or [[beta].sub.1]-nonselective. For example, Ellis et al (14) compared the effect of three doses (50, 100, and 200 mg) of atenolol, a selective [[beta].sub.1]-adrenoceptor-blocking drug, and one dose (40 mg) of propranolol on the airways of 10 asthmatic patients. Two hours after drug administration, the three doses of atenolol were significantly less effective than propranolol in [[beta].sub.2]-blocking, as shown by a smaller FE[V.sub.1] decrease. Moreover, the isoprenaline FE[V.sub.1] dose-response curves gradually shifted to the right of the placebo curve with increasing doses of atenolol, and the greatest displacement was observed with propranolol.
Also [[beta].sub.1]-selective receptor blockers can adversely affect airways at therapeutic oral doses. For example, a 3-week course of atenolol, 100 mg once daily, or metoprolol, 100 mg twice daily, was effective in reducing BP in 14 hypertensive patients with asthma. (18) However, atenolol caused significantly less bronchospasm than metoprolol, less frequent sensations of moderately severe to very severe wheeziness, and more asthma-free days, and had less effect on the evening peak expiratory flow rate (PEFR). This finding suggests that the true clinical relevance of [[beta].sub.1]-adrenoceptor selectivity be re-examined.
All selective [[beta].sub.1]-adrenoceptor antagonists block [[beta].sub.2]-adrenoceptors when their concentrations are high enough, therefore the parenteral route of administration is best avoided. (19) The so-called [[beta].sub.1]-adrenoceptor blockers are not completely [[beta].sub.1]-selective, which could mean that they might have some affinity for [[beta].sub.2]-adrenoceptors on airway smooth muscle. Moreover, the airways may contain functional [[beta].sub.1]-adrenoceptors. (20) Nevertheless, esmolol, a new ultrashort-acting [[beta].sub.1]-selective adrenoceptor blocker, may be preferred over propranolol because of its short duration of action and relative lack of effect on airway resistance in patients with asthma who require treatment with an IV [beta]-adrenoceptor-blocking agent. (21)
Nonselective [beta]-Adrenoceptor-Blocking Agents With Intrinsic Sympathomimetic Activity
The therapeutic importance of intrinsic sympathomimetic activity (ISA), an ancillary pharmacologic property of some [beta]-adrenoceptor-blocking drugs, in asthmatic patients is questionable. Apparently, ISA seems to be at least as important as [[beta].sub.1]-adrenoceptor selectivity in reducing the increase in airway resistance that results from [beta]-adrenoceptor blockade both at rest and during exertion. (22) Moreover, it reduces the bronchoconstrictor response to inhaled histamine during [beta]-adrenoceptor blockade in asthma patients. (23)
Patakas et al (24) demonstrated that propranolol, but not the nonselective [beta]-adrenoceptor-blocking agent pindolol that exerts ISA, significantly affected specific airway conductance and PEFR, although tests of small airway function after pindolol administration showed reduced airflow. Unfortunately, the bronchodilator action of terbutaline on large airways was diminished after the administration of both [beta]-blockers.
Decalmer et al (25) examined the effects of approximately equipotent single oral doses of selective [[beta].sub.1]-adrenoceptor-blocking agents (atenolol, 100 mg; metoprolol, 100 and 300 mg) and four nonselective [beta] ([[beta].sub.1] + [[beta].sub.2])-blockers (propranolol, 100 mg; oxprenolol, 100 mg; timolol, 10 mg; and pindolol, 5 mg). Lindolol is the only agent with ISA on FE[V.sub.1] in 10 asthma patients. All drugs caused a fall in FE[V.sub.1], but only atenolol did not differ significantly from placebo in this respect. Moreover, the four nonselective [beta]-adrenoceptor-blocking drugs blocked the bronchodilator response to inhaled isoprenaline, whereas the selective [[beta].sub.1]-adrenoceptor-blocking agents allowed some bronchodilation.
These observations indicate that pindolol is potentially dangerous in asthmatic patients. In any case, agents with ISA offer less cardioprotection than do [beta]-adrenoceptor blockers without this ancillary property. Therefore, the use of these drugs should be severely restricted.
[[beta].sub.1]-Adrenoceptor Blockers With Mild [[beta].sub.2]-Agonist Properties
The new [beta]-adrenoceptor blockers that have enhanced [[beta].sub.1]-selectivity and partial [[beta].sub.2]-agonist activity affect airway function to a lesser extent than did the earlier blocking agents, but none are considered to be entirely "safe" in patients with asthma. Celiprolol, a [[beta].sub.1]-selective adrenergic-blocking drug with peripheral [[beta].sub.2]-stimulatory, peripheral [[alpha].sub.2]-inhibitory, and partial agonist activities, has been reported to relax bronchial smooth muscle. (26) Isolated tissue studies suggest that a weak [[alpha].sub.2]-blocking effect contributes to this bronchodilation. (26) These findings are encouraging, but clinical experience with these new agents in the treatment of patients with chronic airway obstruction is limited.
In 34 asthmatic patients, propranolol, 40 mg, and to a lesser extent atenolol, 100 mg, but not celiprolol, 200 or 400 mg, caused decreases in FE[V.sub.1] and the midexpiratory phase of forced expiratory flow. (27) Positive changes in FE[V.sub.1] after the administration of each drug plus isoproterenol or albuterol were as follows, in the rank order: celiprolol approximately the same as placebo, greater than atenolol, greater than propranolol. Only propranolol pretreatment caused a significant reduction in the bronchodilator effect.
In normotensive asthmatic patients, the effect of celiprolol, 200 mg or 400 mg, on airways was not significantly different from that of placebo. (28) Conversely, propranolol, 80 mg, caused a significant decrease in FE[V.sub.1] and FVC, and a conspicuous rise in airway resistance compared with placebo. Terbutaline caused further bronchodilation after the administration of celiprolol and placebo, but it did not restore pulmonary function parameters to baseline levels after propranolol administration, even at supratherapeutie doses.
[[beta].sub.1]-Adrenoceptor Blockers That Modulate the Endogenous Production of Nitric Oxide
Nebivolol, a new selective [[beta].sub.1]-adrenoceptor-blocking agent without ISA, modulates the endogenous production of nitric oxide. In particular, nebivolol dilates the human forearm vasculature via the Larginine/nitric oxide pathway. (29) Nebivolol does not significantly decrease airway conductance compared with atenolol or propranolol. (29) In six healthy volunteers, nebivolol, unlike propranolol and atenolol, did not antagonize the effects of albuterol. (30) In 12 asthmatic patients, nebivolol had a slight effect on airway function. (31) However, although its effect on FE[V.sub.1] was statistically significant, the mean percent decrease was small (-8.4%). Nebivolol partially antagonized the bronchodilator response to inhaled albuterol, but the effect was similar to that elicited by celiprolol.
Because of the potential role of nitric oxide in airway control, (32) [[beta].sub.1]-adrenoceptor blockers that modulate the endogenous production of nitric oxide might be a treatment option, but further research is needed to better assess the impact of these drugs on patients with chronic airflow limitation.
Possible Mechanisms of [beta]-Adrenoceptor Blocker-Induced Asthma
The mechanisms of [beta]-adrenoceptor blocker-induced asthma are uncertain. The bronchoconstrictor effects of these drugs seem to be unrelated to [beta]-adrenoceptor blockade in the airway smooth muscle. (33) Marcelle (34) observed that, in healthy subjects and asymptomatic patients, inhaled norepinephrine induced bronchoconstriction and respiratory asynchronism only after pretreatment with low doses of propranolol. Moreover, the airway resistance increases were prevented by phentolamine. These findings indicate that resting bronchial tone is relaxed by the stimulation of [beta]-adrenergic receptor stimulation, while receptor suppression unmasks [alpha]-adrenergic-induced constriction.
It also has been suggested that [beta]-adrenoceptor blockers may inhibit the action of catecholamines on some target cells (eg, airway mast cells) and consequently induce an increase in mediator release. (35) However, no increase in plasma histamine level was detected after IV propranolol administration. (36)
There is some indication that muscarinic [M.sub.2]-receptors, which act as autoreceptors on postganglionic cholinergie nerves and inhibit acetylcholine release, may be defective in asthmatic patients (possibly as a consequence of airway inflammation) and that this may enhance cholinergic reflexes and account for [beta]-adrenoceptor blocker-induced asthma. (37) In fact, [beta]-blockers may inhibit the modulatory effect of circulating epinephrine on [[beta].sub.2]-adrenoceptors of cholinergic nerves, thus increasing acetylcholine release. If there is a deficit in [M.sub.2]-autoreceptors, the increase in acetylcholine cannot switch itself off. The inhibitory effect of oxitropium bromide, an inhaled anticholinergic drug, on [beta]-adrenoceptor blocker-induced asthma supports this theory. (37) Alternatively, [beta]-blockers may increase the release of tachykinins from airway sensory nerves, thereby increasing bronchoconstriction and airway inflammation. (38) Boskabady and Snashall (11) have suggested that antagonism by [beta]-blocking drugs is enhanced in asthmatic patients because these patients are more sensitive to endogenous epinephrine, which may thus dilate and stabilize their airways.
Use of [beta]-Adrenoceptor Blocker in Patients With Airway Dysfunction
Since selective [[beta].sub.1]-adrenoceptor blockers can reduce lung
function, [beta]-adrenoceptor-blocking agents must not be given to patients with airway dysfunction (ie, asthma or COPD), especially those with marked reversibility of airflow. At present, a history of asthma remains a contraindication to the use of any [beta]-adrenergic inhibitor. However, if these agents are thought to confer a substantial benefit in, for example, a patient affected by a bronchospastic disorder after an acute myocardial infarction, then the lowest dose of a selective [[beta].sub.1]-adrenoceptor-blocking drug without ISA (eg, metoprolol, atenolol, or esmolol) that is associated with high doses of a [[beta].sub.2]-agonist may outweigh the risks in some patients with mild intermittent asthma or well-controlled mild persistent asthma (Table 2). (39) Obviously, [[beta].sub.1]-blockers should be administered under direct medical observation. (40)
We think that [beta]-adrenoceptor-blocking agents with [[beta].sub.2]-agonist activity are preferable to conventional [[beta].sub.1]-blockers, although there is still some risk that bronchospasm may occur in certain individuals and that the bronchodilator response to inhaled [[beta].sub.2]-agonists might be impaired with these agents. [[beta].sub.1]-Adrenoceptor blockers, which modulate the endogenous production of nitric oxide, are an interesting option, but more research is needed to better assess the impact of these drugs on patients with chronic airflow limitation.
It must be stressed that the American Academy of Allergy and Immunology warned of a potential increased risk associated with the concomitant administration of allergen immunotherapy and [beta]-blocking agents. (41) The position statement recommended that, when possible, an equally safe and effective drug should substitute for [beta]-blockers. (41)
SELECTIVE [[alpha].sub.1]-ADBENOCEPTOR ANTAGONISTS
Experimental results (49) and clinical results (43) indicate that asthma does not involve significant increases in airway [[alpha].sub.1]-adrenoceptor function. However, such [[alpha].sub.1]-adrenoceptor antagonists as indoramin (44) and phentolamine, (45) sometimes produce an appreciable improvement in lung function. The weak bronchodilation ascribed to [[alpha].sub.1]-antagonists could be explained by other pharmacologic actions of the drugs used. In fact, hybrid drugs, such as indoramin and phentolamine, are [alpha]-adrenoceptor antagonists that exert antihistamine and antiserotonin activities. (46)
Unfortunately, bronchodilation, albeit weak, after the administration of [[alpha].sub.1]-adrenoceptor antagonists has not been confirmed in a few studies. For example, there were no significant differences in pulmonary function tests in a 6-h study (47) in patients with essential hypertension and COPD after dosing with placebo or with prazosin. Moreover, the addition of oral prazosin (2 mg twice daily) to previous antiasthmatic medication for 3 weeks in stable patients with chronic asthma who continued to have symptoms despite conventional treatment did not induce significant changes in PEFR, FE[V.sub.1], FVC, FE[V.sub.1]/FVC ratio, diary card symptom scores, or dose of a [beta]-sympathomimetic agent. (48)
Apparently, selective [[alpha].sub.1]-blockers do not exacerbate preexisting airflow limitations. (49) Therefore, chronic obstruction of airways is not a contraindication to selective [[alpha].sub.1]-blocker prescription.
[[alpha].sub.2]-ADRENOCEPTOR AGONISTS
Human bronchial, cholinergic neurotransmission can be inhibited by the stimulation of prejunctional [[alpha].sub.2]-adrenoceptors. (50) However, the effects of [[alpha].sub.2]-adrenoceptor agonists on the human bronchi may either be harmful or beneficial. In fact, when inhaled, they reduce the immediate bronchial response to allergens, whereas when ingested, they increase the bronchial response to histamine, mainly when their effect on the CNS is greater. (51) Actually, clonidine does not modify the response to histamine in nonasthmatic patients but significantly increases it in asthmatic subjects. (52) On the contrary, it does not significantly affect methacholine-induced bronchoconstriction in asthma patients. (53)
Reports on the effects of [[alpha].sub.2]-adrenoceptor agonists in patients with airway dysfunction are scarce. Deitch et al (54) evaluated the effects of guanabenz on the airways of COPD patients. Only 1 of 64 patients discontinued guanabenz treatment because of asthma exacerbation that was thought to be due to airway dryness. Because of these conflicting findings and the lack of more detailed information, we believe that [[alpha].sub.2]-adrenoceptor agonists should not be used in asthmatic subjects due to their potential harmful effects on the bronchi.
COMBINED [alpha]-ADRENERGIC AND [beta]-ADRENERGIC RECEPTOR BLOCKERS
Labetalol is an antihypertensive agent that combines a nonspecific [[beta].sub.1]-blocker and [[beta].sub.2]-blocker with some [[alpha].sub.1]-blocking activity. These pharmacodynamic actions can affect lung function. In fact one report (55) suggests that the coexistent [[alpha].sub.1]-adrenoceptor blockade does not prevent asthmatic symptoms caused by the [beta]-adrenoceptor blockade. For example, in six healthy male volunteers, the changes in FE[V.sub.1] induced by a 400-mg dose of labetalol did not differ from those of placebo, whereas an 80-mg dose of propranolol reduced resting FE[V.sub.1] and enhanced the fall in FE[V.sub.1] after histamine administration. (56) Moreover, Dal Negro et al (57) showed that three [beta]-adrenoceptor-blocking agents (ie, atenolol, oxprenolol, and metoprolol) caused significant worsening of pulmonary functional parameters in hypertensive patients who also had COPD. On the contrary, labetalol led to a mild improvement in respiratory function.
Carvedilol, a nonselective [beta]-adrenoceptor antagonist that is devoid of ISA and that possesses vasodilator properties secondary to selective [[alpha].sub.1]-adrenoceptor-blocking activity that is considerably weaker than its [beta]-adrenoceptor antagonistic activity, (58) does not elicit any noteworthy effect on pulmonary function. (59) In particular, Guazzi et al (60) have demonstrated that in chronic heart failure, carvedilol ameliorates left ventricular function at rest and does not significantly affect ventilation and pulmonary gas transfer or functional capacity.
Notwithstanding these reports, we believe that the combination of [alpha]-adrenoceptor and [beta]-adrenoceptor blockers is potentially dangerous because the [alpha]-adrenoceptor blockade might fail to prevent the asthmatic symptoms caused by the blockade of [beta]-adrenoceptors.
DIURETICS
Loop diuretics, including the clinically efficacious agents bumetanide and furosemide, (61) and thiazides (62) inhibit Na-K-Cl cotransport activity. The Na-K-Cl cotransporters are a class of membrane proteins that transport Na, K, and Cl ions into and out of cells in an electrically neutral manner. Cotransporter regulation is highly tissue-specific, which perhaps in part is related to the presence of different Na-K-Cl cotransporter isoforms. (63) The Na-K-Cl cotransporter mediates both [K.sup.+] influx and efflux in airway smooth muscle. Na-K pump inhibition stimulates outward Na-K-Cl cotransport as a result of an increase in intracellular [Na.sup.+] content.
These findings explain why thiazide diuretics do not have significant adverse effects on airway function and may be considered the agents of choice for initial therapy in patients with asthma. On the contrary, loop diuretics are generally not considered for use as antihypertensive medication because their effect on reducing peripheral vascular resistance is smaller when compared to thiazide diuretics.
However, diuretics may interfere with mucus production. (64) Moreover, thiazide diuretics must be used with caution in hypertensive patients with COPD. In fact, being potassium-wasting diuretics, they may worsen C[O.sub.2] retention in hypoventilating patients and potentiate hypokalemia in those patients receiving corticosteroids. In addition, [beta]-agonists may substantially lower serum potassium levels in patients already rendered hypokalemic by diuretics.
Patients with COPD receiving potassium-wasting diuretics who have chronic respiratory acidosis or are receiving corticosteroids or [beta]-agonists should undergo close monitoring of electrolyte levels and be considered for therapy with potassium supplements or, preferably, potassium-sparing agents. (65) However, although thiazides are potentially dangerous in patients with COPD, significantly fewer adverse effects have been reported with these diuretics since the finding that low doses can be clinically beneficial.
CALCIUM CHANNEL BLOCKERS
Calcium plays a central role in the activation of many cellular processes, including contraction, which is the most relevant end point in airway smooth muscle physiology. (66) Although the maintenance of tone may depend on some calcium entry, this may be also via channels that are not sensitive to conventional calcium antagonists. (35) Similarly, the release of mediators from inflammatory cells is not mediated via voltage-dependent calcium channels, and there is no evidence that calcium antagonists have a significant effect on mediator release, the activation of inflammatory cells, or the process of inflammation. (35)
Nevertheless, some calcium channel blockers may have beneficial or neutral effects in hypertensive patients with asthma or COPD because they can alter nonspecific airway hyperreactivity. For example, Kivity et al (67) observed that nifedipine (20 mg), but not diltiazem (60 mg), significantly raised the provocative concentration of methacholine causing a 20% fall in FE[V.sub.1] (P[C.sub.20]) in asthmatic patients when compared with placebo. When diltiazem was given alone, it had no effect on the airway but, when given with nifedipine, it significantly raised the P[C.sub.20]. This effect was superior to that of any of the other treatments that were given. It has been suggested (68) that nifedipine exerts an effect principally on mediators that are dependent on external calcium sources, such as methacholine, for stimulus-contraction coupling in the airways. However, nifedipine also inhibited the exercise fall in FE[V.sub.1]. (69) It is noteworthy that nifedipine induces a small potentiation of [[beta].sub.2]-adrenoceptor-mediated bronchodilation, which is important when treating patients affected by both asthma and hypertension. (70)
Calcium channel blockers may be given to hypertensive patients who are affected by airway disease instead of other harmful antihypertensive agents because they do not adversely affect the airways. They may be a good alternative to [[beta].sub.2]-adrenergic antagonists in that they may also possess some bronchodilating effects.
ACE INHIBITORS
Whereas ACE inhibition is safe in the vast majority of patients with obstructive airway disease, asthmatic symptoms, the exacerbation of asthma, and a rise in bronchial reactivity occasionally have been reported. (71) A dry cough, which may not necessarily occur shortly after the institution of therapy but perhaps months or even a year later, is the most common adverse effect of ACE inhibitors. (72) Cough has emerged as a drug class effect of all ACE inhibitors. (73) Controlled studies (74) have suggested that the incidence may be as high as 20%, and the problem seems to occur much more often in women. No links with sex, history of bronchospasm, drug type, or drug dose have been identified, although ACE expression is decreased in the epithelium of asthmatic patients and is associated with increased eosinophil inflammation. (75)
ACE inhibitor-induced cough and bronchospasm are probably linked to the suppression of kininase II activity. ACE catalyzes the conversion of angiotensin I into angiotensin II. It also inhibits the action of kininase II, which may lead to an accumulation of bradykinin (Fig 2) and substance P in the lung. Bradykinin may induce cough and bronchospasm in susceptible persons by stimulating sensory C-fibers and phospholipase [A.sub.2], which increases the production of arachidonic acid metabolites and prostaglandins (PGs), especially PG[E.sub.2] and PG[I.sub.2]. Substance P, being a neurotransmitter for C-fibers, produces bronchoconstriction.
[FIGURE 2 OMITTED]
Replacement with another ACE inhibitor should not be tried. Data suggest that a person experiencing a cough with one ACE inhibitor will most likely experience cough with the others. The cough usually will clear within a month after the withdrawal of the ACE inhibitor. (74)
Since ACE inhibitors may cause dry cough during treatment, and occasionally may worsen or even induce asthma, they could be hazardous in asthmatic patients. In any case, in patients who develop cough after the assumption of an ACE inhibitor, theophylline (76) or cromolyn sodium (77) therapy can reduce the extent of the symptom.
A[T.sub.1] RECEPTOR ANTAGONISTS
A[T.sub.1]-antagonists (eg, losartan, valsartan, irbesartan, or candesartan) are recommended as an alternative for patients at risk for cough while receiving ACE inhibitors. (78) In fact, these drugs, unlike ACE inhibitors, are selective and do not block the effects of kininase II, so they neither lead to the accumulation of bradykinin or substance P (Fig 2) nor enhance PG synthesis. (79) A study (80) of A[T.sub.1]-blockers suggested that cough has a similar prevalence in patients taking the drug (prevalence, 2.8 to 3.4%) and patients taking placebo. However, in a randomized study, (80) losartan induced cough in 9.9% of subjects.
There are several reports (81-83) of cases of losartan-induced bronchoconstriction that may result from the inhibition of endogenous nitric oxide release in the airway. (84) This finding was unexpected because it is well-known that IV angiotensin II causes bronchoconstriction in patients with mild asthma (85) and potentiates methacholine-induced bronchoconstriction both in vitro and in vivo, (86) although it has no effect on histamine-evoked bronchoconstriction in human bronchi in vitro or in vivo (87) and it does not potentiate endothelin-1-induced bronchoconstriction at sub-bronchoconstrictor doses in asthma patients. (88) Furthermore, the renin-angiotensin system is activated in a subpopulation of asthmatic patients during acute attacks of severe asthma, although the mechanism of this activation remains unclear. (89) Since angiotensin II levels are elevated in these patients, a beneficial effect of A[T.sub.1]-antagonists is possible. Recently, Myou et al (90) demonstrated that bronchial hyperresponsiveness to methacholine, in terms of a provocative concentration of methacholine causing a 35% fall in standardized partial expiratory flow at 40% of FVC but not P[C.sub.20] FE[V.sub.1], was attenuated by losartan in eight asthmatic patients.
A[T.sub.1]-antagonists seem to be reasonable alternatives for patients with ACE-inhibitor cough. However, clinical experience is very limited, and, consequently, there is no detailed information regarding adverse reactions to A[T.sub.1]-antagonists. Moreover, according to some reports, A[T.sub.1]-receptor antagonists may not be entirely free of ACE inhibitor-related side effects.
5-HYDROXYTRYPTAMINE MODIFIERS
5-Hydroxytryptamine (5-HT) induces a bimodal response in human tracheal and bronchial tissue, which reflects the effect of two 5-HT receptor sub-types. (91) The direct contractile effects of 5-HT are probably mediated through 5-HT2A sites on smooth muscle, whereas the relaxant effects are probably due to the activation of 5-HT1A receptors (Fig 3). (92,93)
[FIGURE 3 OMITTED]
Ketanserin is a 5-HT2A receptor-blocking agent that is marketed in several European countries but not in the United States. It is particularly indicated in elderly hypertensive patients, especially in those with peripheral vascular disease. However, the decrease in BP induced by ketanserin is smaller than that observed after administration of nifedipine, enalapril, sodium nitroprusside, dihydralazine, and urapidil, (94) which is a hybrid 5-HT modifier.
One study (95) has shown that ketanserin produces mild bronchodilation in patients with chronic obstruction of the airways when administered IV in a 10-mg dose. IV ketanserin has a rapid onset of action and induces a more prolonged bronchial response than does inhaled ketanserin. (95) Ketanserin, which has been tested in asthma patients, had only a marginal effect on methacholine-induced bronchospasm. (96) However, it attenuated adenosine-induced bronchoconstriction but did not inhibit histamine-induced bronchoconstriction in adult asthmatic subjects. (97) Moreover, ketanserin did not attenuate exercise-induced bronchoconstriction in adult asthma patients (98) or in children with atopic asthma. (99)
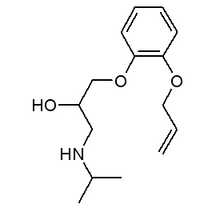
Urapidil is a peripheral postsynaptic [[alpha].sub.1]-adrenoceptor antagonist that exerts central agonistic action at 5-HTIA receptors. This antihypertensive agent exerts beneficial effects on pulmonary and cardiac hemodynamics when administered IV or orally to COPD patients with secondary pulmonary hypertension or cor pulmonale. (100) Furthermore, the IV administration of urapidil caused a moderate bronchodilation in patients with obstructive airway disease. (101)
CONCLUSION
Not all antihypertensive drugs should be used to treat uncomplicated systemic arterial hypertension in patients with concurrent airway dysfunction (Table 3). Based on the reviewed literature, we suggest that thiazide diuretics or calcium channel blockers be prescribed for these patients (Fig 4) because the majority of cases of hypertension can usually be controlled by one drug, even if it is not the first agent chosen for treatment. For many patients, however, a single drug fails to produce the recommended or optimal BP levels. (1) In that case, managing hypertensive patients who have asthma or COPD requires constant supervision of medication usage and a careful review of the entire list of medications at each presentation. In any case, every effort should be made to reduce the possibility of deleterious effects on airways.
[FIGURE 4 OMITTED]
ACKNOWLEDGMENT: We are grateful to Dr. Jean Ann Gilder for revising the text.
REFERENCES
(1) Kendall MJ, Toescu V. The HOT study: hypertension optimal therapy. J Clin Pharm Ther 1998; 23:137-139
(2) National Institutes of Health. The Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, MD: National Heart, Lung and Blood Institute of the National Institutes of Health; November 1997; NIH Publication No. 98-4080
(3) World Health Organization and International Society of Hypertension. 1999 World Health Organization-International Society of Hypertension guidelines for the management of hypertension: guidelines subcommittee. J Hypertens 1999; 17:151-183
(4) Antonelli Incalzi R, Fuso L, De Rosa M, et al. Co-morbidity contributes to predict mortality of patients with chronic obstructive pulmonary disease. Eur Respir J 1997; 10:2794-2800
(5) Lofdahl CG. Antihypertensive drugs and airway function, with special reference to calcium channel blockade. J Cardiovasc Pharmacol 1989; 14(suppl):S40-S51
(6) Carstairs JR, Nimmo AJ, Barnes PJ. Autoradiographic visualization of [beta]-adrenoceptor subtypes in human lung. Am Rev Respir Dis 1985; 132:541-547
(7) Ruffin RE, McIntyre EL, Latimer KM, et al. Assessment of [beta]-adrenoceptor antagonists in asthmatic patients. Br J Clin Pharmacol 1982; 13(suppl):325S-335S
(8) van Herwaarden CL. [beta]-Adrenoceptor blockade and pulmonary function in patients suffering from chronic obstructive lung disease. J Cardiovasc Pharmacol 1983; 5(suppl):S46-S50
(9) Dunn TL, Gerber MJ, Shen AS, et al. The effect of topical ophthalmic instillation of timolol and betaxolol on lung function in asthmatic subjects. Am Rev Respir Dis 1986; 133:264-268
(10) Beumer HM. Adverse effects of [beta]-adrenergic receptor blocking drugs on respiratory function. Drugs 1974; 7:130-138
(11) Boskabady MH, Snashall PD. Bronchial responsiveness to [beta]-adrenergic stimulation and enhanced beta-blockade in asthma. Respirology 2000; 5:111-118
(12) Schwartz S, Davies S, Juers JA. Life-threatening cold and exercise-induced asthma potentiated by administration of propranolol. Chest 1980; 78:100-101
(13) Astrom H. Comparison of the effects on airway conductance of a new selective [beta]-adrenergic blocking drug, atenolol, and propranolol in asthmatic subjects. Scand J Respir Dis 1975; 56:292-296
(14) Ellis ME, Sahay JN, Chatterjee SS, et al. Cardioselectivity of atenolol in asthmatic patients. Eur J Clin Pharmacol 1981; 21:173-176
(15) Singh BN, Whitlock RM, Comber RH, et al. Effects of cardioselective [beta] adrenoceptor blockade on specific airways resistance in normal subjects and in patients with bronchial asthma. Clin Pharmacol Ther 1976; 19:493-501
(16) Lofdahl CC, Dahlof C, Westergren G, et al. Controlled-release metoprolol compared with atenolol in asthmatic patients: interaction with terbutaline. Eur J Clin Pharmacol 1988; 33(suppl):S25-S32
(17) Lawrence DS, Sahay JN, Chatterjee SS, et al. Asthma and [beta]-blockers. Eur J Clin Pharmacol 1982; 22:501-509
(18) Braat MC, Jonkers RE, van Boxtel CJ. Quantification of metoprolol [[beta].sub.2]-adrenoceptor antagonism in asthmatic patients by pharmacokinetic-pharmacodynamic modeling. Pulm Pharmacol 1992; 5:31-38
(19) Wood AJ. Pharmacologic differences between [beta] blockers. Am Heart J 1984; 108:1070-1077
(20) Lammers JW, Folgering HT, van Herwaarden CL. Respiratory tolerance of bisoprolol and metoprolol in asthmatic patients. J Cardiovasc Pharmacol 1986; 8(suppl):S69-S73
(21) Sheppard D, DiStefano S, Byrd RC, et al. Effects of esmolol on airway function in patients with asthma. J Clin Pharmacol 1986; 26:169-174
(22) Taylor SH. Intrinsic sympathomimetic activity: clinical fact or fiction? Am J Cardiol 1983; 52:16D-26D
(23) Dorow P. Influence of intrinsic sympathomimetic activity (ISA) during [beta]-adrenoceptor blockade in asthmatics. Br J Clin Pharmacol 1982; 13(suppl):321S-323S
(24) Patakas D, Argiropoulou V, Louridas G, et al. [beta]-blockers in bronchial asthma: effect of propranolol and pindolol on large and small airways. Thorax 1983; 38:108-112
(25) Decalmer PB, Chatterjee SS, Cruickshank JM, et al. [beta]-blockers and asthma. Br Heart J 1978; 40:184-189
(26) Pruss TP, Khandwala A, Wolf PS, et al. Celiprolol: a new [beta] adrenoceptor antagonist with novel ancillary properties. J Cardiovasc Pharmacol 1986; 8(suppl):S29-S32
(27) Doshan HD, Rosenthal RR, Brown R, et al. Celiprolol, atenolol and propranolol: a comparison of pulmonary effects in asthmatic patients. J Cardiovasc Pharmacol 1986; 8(suppl):S105-S108
(28) Matthys H, Doshan HD, Ruhle KH, et al. The bronchosparing effect of celiprolol, a new [[beta].sub.1]-[[alpha.sub.2]-receptor antagonist on pulmonary function of propranolol-sensitive asthmatics. J Clin Pharmacol 1985; 25:354-359
(29) Mangrella M, Rossi F, Fici F, et al. Pharmacology of nebivolol. Pharmacol Res 1998; 38:419-431
(30) Mohammed AF, Hulks G, Thomson NC, et al. Effects of nebivolol, atenolol and propranolol on airway [beta]-adrenergic responsiveness in normal subject. Clin Drug Investig 1991; 3(suppl):196-198
(31) Cazzola M, Noschese P, D'Amato M, et al. Comparison of the effects of single oral doses of nebivolol and celiprolol on airways in patients with mild asthma. Chest 2000; 118:1322-1326
(32) Matera MG. Nitric oxide and airways. Pulm Pharmacol Ther 1998; 11:341-348
(33) Ney UM. Propranolol-induced airway hyperreactivity in guinea-pigs. Br J Pharmacol 1983; 79:1003-1009
(34) Marcelle R. [alpha]-Adrenergic bronchoconstriction in man. Arch Physiol Biochem 1996; 104:851-854
(35) Barnes BJ, Thomson NC. Other therapies used in asthma. In: Barnes BJ, Rodger IW, Thomson NC, eds. Asthma: basic mechanisms and clinical management. 2nd ed. London, UK: Academic Press, 1992; 659-666
(36) Ind PW, Barnes PJ, Brown MJ, et al. Plasma histamine concentration during propranolol induced bronchoconstriction. Thorax 1985; 40:903-909
(37) Barnes PJ. Muscarinic receptor subtypes in airways. Life Sci 1993; 52:521-527
(38) Kamikawa Y, Shimo Y. Inhibitory effects of catecholamines on cholinergically and non-cholinergically mediated contractions of guinea-pig isolated bronchial muscle. J Pharm Pharmacol 1990; 42:131-134
(39) Chafin CC, Soberman JE, Demirkan K, et al. [beta]-Blockers after myocardial infarction: do benefits ever outweigh risks in asthma? Cardiology 1999; 92:99-105
(40) Tafreshi MJ, Weinacker AB. [beta]-Adrenergic-blocking agents in bronchospastic diseases: a therapeutic dilemma. Pharmacotherapy 1999; 19:974-978
(41) Kaplan AP, Anderson JA, Valentine MD, et al. [beta]-adrenergic blockers, immunotherapy, and skin testing: American Academy of Allergy and Immunology. J Allergy Clin Immunol 1989; 84:129-130
(42) Spina D, Rigby PJ, Paterson JW, et al. [[alpha].sub.1]-Adrenoceptor function and autoradiographic distribution in human asthmatic lung. Br J Pharmacol 1989; 97:701-708
(43) Barnes PJ, Ind PW, Dollery CT. Inhaled prazosin in asthma. Thorax 1981; 36:378-381
(44) Black JL, Temple DM, Anderson SD. Long-term trial of an [alpha]-adrenoceptor blocking drug (Indoramin) in asthma: a preliminary report. Scand J Respir Dis 1978; 59:307-312
(45) Van Mieghem W, Stevens E, Billiet L. Phentolamine therapy in severe chronic asthmatiform bronchitis. Respiration 1981; 42:184-187
(46) Hoffman BB, Lefkowitz RJ. Catecholamines, sympathomimetic drugs, and adrenergic receptor antagonists. In: Hardman JG, Limbird LE, Molinoff PB, et al, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996; 199-248
(47) Chodosh S, Tuck J, Pizzuto D. Prazosin in hypertensive patients with chronic bronchitis and asthma: a brief report. Am J Med 1989; 86:91-93
(48) Baudouin SV, Aitman TJ, Johnson AJ. Prazosin in the treatment of chronic asthma. Thorax 1988; 43:385-387
(49) Biernacki W, Flenley DC. Doxazosin, a new [alpha]-1-antagonist drug, controls hypertension without causing airways obstruction in asthma and COPD. J Hum Hypertens 1989; 3:419-425
(50) Grundstrom N, Andersson RG. Inhibition of the cholinergic neurotransmission in human airways via prejunctional [[alpha].sub.2]-adrenoceptors. Acta Physiol Scand 1985; 125:513-517
(51) Dinh Xuan AT, Lockhart A. Bronchial effects of [[alpha].sub.2]-adrenoceptor agonists and of other antihypertensive agents in asthma. Am J Med 1989; 87:34S-37S
(52) Dinh Xuan AT, Regnard J, Matran R, et al. Effects of clonidine on bronchial responses to histamine in normal and asthmatic subjects. Eur Respir J 1988; 1:345-350
(53) Foxworth JW, Reisz GR, Pyszczynski DR, et al. Oral clonidine in patients with asthma: no significant effect on airway reactivity. Eur J Clin Pharmacol 1995; 48:19-22
(54) Deitch MW, Littman GS, Pascucci VL. Antihypertensive therapy with guanabenz in patients with chronic obstructive pulmonary diseases. J Cardiovasc Pharmacol 1984; 6(suppl): S818-S822
(55) Larsson K. Influence of labetalol, propranolol and practolol in patients with asthma. Eur J Respir Dis 1982; 63:221-230
(56) Maconochie JG, Woodings EP, Richards DA. Effects of labetalol and propranolol on histamine-induced bronchoconstriction in normal subjects. Br J Clin Pharmacol 1977; 4:157-162
(57) Dal Negro RW, Zoccatelli O, Pomari C, et al. Respiratory effects of four adrenergic blocking agents combined with a diuretic in treating hypertension with concurrent chronic obstructive lung disease. Int J Clin Pharmacol Res 1986; 6:283-289
(58) van Zwieten PA. Pharmacodynamic profile of carvedilol. Cardiology 1993; 82(suppl):19-23
(59) Sundberg S, Tiihonen K, Gordin A. Vasodilatory effects of carvedilol and pindolol. J Cardiovasc Pharmacol 1987; 10(suppl):S76-S80
(60) Guazzi M, Agostoni P, Matturri M, et al. Pulmonary function, cardiac function, and exercise capacity in a follow-up of patients with congestive heart failure treated with carvedilol. Am Heart J 1999; 138:460-467
(61) Rhoden KJ, Douglas JS. Evidence of Na-K-Cl cotransport in airway smooth muscle. Am J Physiol 1995; 268:L551-L557
(62) Mastroianni N, De Fusco M, Zollo M, et al. Molecular cloning, expression pattern, and chromosomal localization of the human Na-Cl thiazide-sensitive cotransporter (SLC12A3). Genomics 1996; 35:486-493
(63) Haas M. The Na-K-Cl cotransporters. Am J Physiol 1994; 267:C869-C885
(64) Krane NK, Wallin JD. Managing the elderly patient with both hypertension and pulmonary disease. Geriatrics 1987; 42:45-49
(65) Hill NS. Fluid and electrolyte considerations in diuretic therapy for hypertensive patients with chronic obstructive pulmonary disease. Arch Intern Med 1986; 146:129-133
(66) Janssen LJ. Calcium handling in airway smooth muscle: mechanisms and therapeutic implications. Can Respir J 1998; 5:491-498
(67) Kivity S, Brayer M, Topilsky M. Combined effect of nifedipine and diltiazem on methacholine-induced bronchoconstriction in asthmatic patients. Ann Allergy 1992; 68:175-179
(68) Henderson AF, Costello JF. The effect of nifedipine on bronchial reactivity to inhaled histamine and methacholine: a comparative study in normal and asthmatic subjects. Br J Dis Chest 1988; 82:374-381
(69) Crimi N, Palermo F, Sorace R, et al. Effect of a calcium antagonist, nifedipine, in exercise-induced asthma. Respiration 1984; 45:262-264
(70) Svedmyr K, Lofdahl CG, Svedmyr N. Nifedipine-a calcium channel blocker-in asthmatic patients: interaction with terbutaline. Allergy 1984; 39:17-22
(71) Wood R. Bronchospasm and cough as adverse reactions to the ACE inhibitors captopril, enalapril and lisinopril: a controlled retrospective cohort study. Br J Clin Pharmacol 1995; 39:265-270
(72) Overlack A. ACE inhibitor-induced cough and bronchospasm: incidence, mechanisms and management. Drug Saf 1996; 15:72-78
(73) Roisman GL, Danel CJ, Lacronique JG, et al. Decreased expression of angiotensin-converting enzyme in the airway epithelium of asthmatic subjects is associated with eosinophil inflammation. J Allergy Clin Immunol 1999; 104:402-410
(74) McEwan JR, Fuller RW. Angiotensin converting enzyme inhibitors and cough. J Cardiovasc Pharmacol 1989; 13(suppl):S67-S69
(75) Semple PF. Putative mechanisms of cough after treatment with angiotensin converting enzyme inhibitors. J Hypertens 1995; 13(suppl):S17-S21
(76) Cazzola M, Matera MG, Liccardi G, et al. Theophylline in the inhibition of angiotensin-converting enzyme inhibitor-induced cough. Respiration 1993; 60:212-215
(77) Hargreaves M. Sodium cromoglycate: a remedy for ACE inhibitor-induced cough. Br J Clin Pract 1993; 47:319-320
(78) Waeber B, Burnier M, Nussberger J, et al. Experience with angiotensin II antagonists in hypertensive patients. Clin Exp Pharmacol Physiol 1996; 3(suppl):S142-S146
(79) Kirk JK. Angiotensin-II receptor antagonists: their place in therapy. Am Fam Physician 1999; 59:3140-3148
(80) Lacourciere Y, Brunner H, Irwin R, et al. Effects of modulators of the renin-angiotensin-aldosterone system on cough. J Hypertens 1994; 12:1387-1393
(81) Dicpinigaitis PV, Thomas SA, Sherman MB, et al. Losartan-induced bronchospasm. J Allergy Clin Immunol 1996; 98: 1128-1130
(82) Anonymous. Bronkospasm och hosta kopplade till Losartan. Lakartidningen 1995; 92:3920
(83) Conigliaro RL, Gleason PP. Losartan-induced cough after lisinopril therapy. Am J Health Syst Pharm 1999; 56:914-915
(84) Kanazawa H, Hirata K, Yoshikawa J. Guinea pig airway hyperresponsiveness induced by blockade of the angiotensin II type 1 receptor: role for endogenous nitric oxide. Am J Respir Crit Care Med 1999; 159:165-168
(85) Millar EA, Angus RM, Hulks G, et al. Activity of the renin-angiotensin system in acute severe asthma and the effect of angiotensin II on lung function. Thorax 1994; 49:492-495
(86) Millar EA, Nally JE, Thomson NC. Angiotensin II potentiates methacholine-induced bronchoconstriction in human airway both in vitro and in vivo. Eur Respir J 1995; 8:1838-1841
(87) Ramsay SG, Clayton RA, Dagg KD, et al. Effect of angiotensin II on histamine-induced bronchoconstriction in the human airway both in vitro and in vivo. Respir Med 1997; 91:609-615
(88) Chalmers GW, Millar EA, Little SA, et al. Effect of infused angiotensin II on the bronchoconstrictor activity of inhaled endothelin-1 in asthma. Chest 1999; 115:352-356
(89) Ramsay SG, Dagg KD, McKay IC, et al. Investigations on the renin-angiotensin system in acute severe asthma. Eur Respir J 1997; 10:2766-2771
(90) Myou S, Fujimura M, Kamio Y, et al. Effect of losartan, a type 1 angiotensin II receptor antagonist, on bronchial hyperresponsiveness to methacholine in patients with bronchial asthma. Am J Respir Crit Care Med 2000; 162:40-44
(91) Cazzola M, Matera MG. 5-HT modifiers as a potential treatment of asthma. Trends Pharmacol Sci 2000; 21:13-16
(92) Cazzola M, Matera MG, D'Amato G, et al. Effects of serotonin on airways: recent developments. Allergy 1995; 50:1-10
(93) Cazzola M, D'Amato G, Lobefalo G, et al. Ketanserin, a new blocking agent of serotonin [S.sub.2]-receptors: respiratory functional effects in chronic obstruction of the airways. Chest 1987; 92:863-866
(94) Dooley M, Goa KL Urapidil. A reappraisal of its use in the management of hypertension. Drugs 1998; 56:929-955
(95) Cazzola M, Guidetti E, Sepe J, et al. Acute respiratory and cardiovascular effects of inhaled ketanserin in chronic obstructive pulmonary disease: a comparative study with intravenously administered ketanserin. Chest 1990; 97:901-905
(96) Cazzola M, Assogna G, Lucchetti G, et al. Effect of ketanserin, a new blocking agent of the 5-H[T.sub.2] receptor, on airway responsiveness in asthma. Allergy 1990; 45:151-153
(97) Cazzola M, Matera MG, Santangelo G, et al. Effect of the selective 5-H[T.sub.2] antagonist ketanserin on adenosine-induced bronchoconstriction in asthmatic subjects. Immunopharmacology 1992; 23:21-28
(98) Stott DJ, Roberts JA, Thomson NC, et al. The effects of the 5 H[T.sub.2] antagonist ketanserin in adult atopic asthma. Eur J Clin Pharmacol 1988; 35:209-212
(99) So SY, Lam WK, Kwan S. Selective 5-H[T.sub.2] receptor blockade in exercise-induced asthma. Clin Allergy 1985; 15:371-376
(100) Adnot S, Samoyeau R, Weitzenblum E. Treatment of pulmonary hypertension in patients with chronic obstructive pulmonary disease: position of vasodilators with special focus on urapidil. Blood Press Suppl 1995; 3:47-57
(101) Cazzola M, Spinazzi A, Santangelo G, et al. Acute effects of urapidil on airway response in hypertensive patients with chronic obstructive pulmonary disease. Drugs 1990; 40(suppl):71-72
* From the Dipartimento di Pneumologia (Drs. Cazzola, Noschese, and D'Amato), Unita Operativa Complessa di Pneumologia ed Allergologia, Ospedale A. Cardarelli, Napoli, Italy; and the Dipartimento di Medicina Sperimentale (Dr. Matera), Facolta di Medicina e Chirurgia, Seconda Universita Napoletana, Napoli, Italy
Dr. Cazzola has been reimbursed by the following companies for speaking at educational symposiums, consultancy work, research funding, or attending scientific meetings: GlaxoSmithKline, AstraZeneca, Novartis Pharma, Eli Lilly, Bayer, Menarini Farmaceutici, Chiesi Farmaceutici, Abbott, Lusofarmaco, Malesci. Dr. D'Amato has been reimbursed by the following companies for speaking at educational symposiums, consultancy work, research finding, or attending scientific meetings: GlaxoSmithKline, AstraZeneca, Novartis Pharma, Menarini Farmaceutici, Boehringer Ingelheim, Shering Plough, Gentili, Aventis.
Manuscript received June 1, 2000; revision accepted May 17, 2001.
Correspondence to: Mario Cazzola, MD, FCCP, Via del Parco Margherita 24, 80121 Napoli, Italy; e-mail: mcazzola@qubisoft.it
COPYRIGHT 2002 American College of Chest Physicians
COPYRIGHT 2002 Gale Group