Oxycodone
Oxycodone is a very powerful and potentially addictive opioid analgesic medication synthesized from thebaine. more...
It is effective orally and is marketed in combination with aspirin (Percodan, Endodan, Roxiprin) or acetaminophen (Percocet, Endocet, Roxicet, Tylox) for the relief of pain. More recently, ibuprofen has been added to oxycodone (Combunox). It is also sold in a sustained-release form by Purdue Pharma under the trade name OxyContin as well as generic equivalents, and instant-release forms OxyIR, OxyNorm and Percolone . OxyContin is available in 10, 20, 40, and 80 mg tablets, and, due to its sustained-release mechanism, is effective for eight to twelve hours. (The 160 mg formulation was discontinued in May 2001.) OxyNorm is available in 5, 10, and 20 mg capsules and tablets; also as a 1 mg/1 ml liquid in 250 mg bottles and as a 10 mg/1 ml concentrated liquid in 100 mg bottles.
In the United States, oxycodone is a Schedule II controlled substance both as a single agent and in combination products containing acetaminophen or aspirin.
Chemical structure
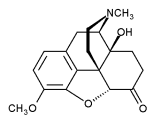
The chemical structure of oxycodone is the methylether of oxymorphone: 3-Methyl-oxymorphone. It could be also described as 14-Hydroxy-Codeinone. It is principally supplied as its hydrochloride salt: oxycodone hydrochloride
Medical use
Oxycodone is one of the most powerful medications for pain control that can be taken orally. Percocet tablets (oxycodone with acetaminophen) are routinely prescribed for post-operative pain control. Oxycodone is also used in treatment of moderate to severe chronic pain. When used at recommended doses for relatively short periods (several weeks), it provides effective pain control with manageable side effects.
Nausea, constipation, lightheadedness, rash, dizziness, and emotional mood disorders are the most frequently reported side effects.
Tolerance and physical dependence occurs after several months of treatment, with larger doses being required to achieve the same degree of analgesia.
According to the DEA and the companies that manufacture the drug, psychological addiction as a result of medical use is extremely rare. However, there are several lawsuits underway brought by plaintiffs who claim that they became addicted to the drug as a result of medical use.
Abuse
The introduction of OxyContin in 1995 resulted in increasing patterns of abuse. Unlike Percocet, whose potential for abuse is limited by the presence of acetaminophen, OxyContin contains only oxycodone and inert filler. Abusers crush the tablets to defeat the time-release mechanism, then either ingest the resulting powder orally, intranasally, via intravenous/intramuscular/subcutis injection, or rectally to achieve rapid absorption into the bloodstream. The vast majority of OxyContin-related deaths are attributed to ingesting substantial quantities of OxyContin or ingesting OxyContin along with another depressant of the central nervous system such as alcohol or benzodiazepines. While high doses of oxycodone can be fatal to an opiate-naïve individual in and of itself, this is (comparatively) rarely the case. It was once felt that "combination" opioids (those that contain one or more additional, non-narcotic ingredients) would be less subject to abuse, since, for example, the amount of acetaminophen present in large overdoses of Percocet would cause stomach upset and liver damage. However, it has been demonstrated that abusers seeking the euphoric "high" are not deterred by these potential side effects or toxicities. Abusers soon discovered that extremely simple methods to separate the ingredients exist, particularly due to the widely disparate solubility of the alkaloids and analgesics in water ("cold water extraction").
Read more at Wikipedia.org