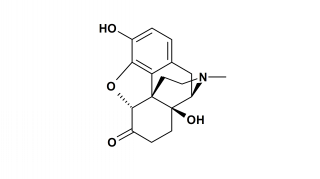
Oxymorphone
Oxymorphone is a powerful synthetic narcotic analgesic drug that is similar to morphine. Clinically, it is administered either via injection or suppository, typically in dosages of 1mg (injected) to 5mg (suppository). As a narcotic, oxymorphone can be habit forming, leading to addiction. more...
Chemical Structure
The chemical structure of Oxymorphone could be also described as 14-Hydroxydihydromorphinone.
Usage
In some cases, it is used during pregnancy. Some veterinarians use the drug during animal operations, such as spaying and neutering or declawing.
A semi-synthetic phenanthrene narcotic agonist, oxymorphone HCL occurs as odorless white crystals or white to off-white powder. It will darken in color with prolonged exposure to light. One gram of oxymorphone is soluble in 4ml of water and it is slightly soluble in alcohol and ether. The commercially available injection has a pH of 2.7-4.5.
Legal Status
Oxymorphone is a C-II controlled substance.
Medicaments
Injectable opioid sedative/restraining agent, analgesic and preanesthetic
DuPont markets oxymorphone under the trade name Numorphan.
Read more at Wikipedia.org