Abstract
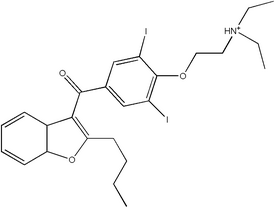
Amiodarone is commercially available as both a tablet and an injectable formulation. It is widely dispensed as an extemporaneously compounded suspension for pediatric and geriatric patients. Extensive stability data at numerous temperatures and a calculated shelf life based on kinetics for such a formulation have not been reported. Refrigeration and room temperature data for finite times have been reported in the literature.
In this study, a stable extemporaneous formulation of amiodarone hydrochloride was formulated using Pacerone tablets. The formulation consisted of 0.75% carboxymethylcellulose and 0.75% Veegum as suspending agents; 25% sucrose, provided as simple syrup; and aqueous strawberry concentrate as flavor. A total of 2.5 L of the formulation was compounded and stored in quantities of 150 mL in 8-oz glass containers. The initial drug content was determined by reverse-phase high-performance liquid chromatography, with a method that was developed in our laboratory. The stability study was carried out by storing three containers at five different temperatures, namely 4, 30, 40, 50 and 60 deg C. The suspension was analyzed at the end of 2, 6, 12, 24 and 48 hours at 7 days, and, thereafter, at the end of every week for 13 weeks. The percentage of drug remaining was plotted against time for each temperature. The slope of the regression line was obtained for each temperature and the zero-order degradation rate constant obtained. The logarithm of the zero-order degradation rate constants was plotted against the inverse of the temperature in degrees Kelvin to obtain the Arrhenius plot. From the regression line for the Arrhenius plot, the zero-order degradation constant at 25 deg C was calculated to be 0.0517 day^sup -1^. The shelf life for the formulation at 25 deg C was calculated to be 193.4 days; the shelf life under refrigeration (4 deg C) was found to be 677.3 days. The degradation products were characterized using high-performance liquid chromatographic-mass spectrometry. Data obtained from the analysis suggested that amiodarone cleaved at its ketone and ether linkages to yield corresponding degradation products.
Introduction
Amiodarone is one of the most powerful anti-arrhythmic drugs used in the treatment of ventricular and supraventricular tachycardias, including those associated with Wolff-Parkinson-White (WPW) syndrome.1,2 Amiodarone is a class III anti-arrhythmic agent that acts by delaying repolarization and prolonging the action-potential duration of atrial and ventricular muscles without altering the resting membrane potential. Amiodarone thus lengthens the refractory period of the atrial and ventricular myocardia, atrioventricular node and accessory pathways that mediate WPW syndrome.3
The drug is commercially available as tablet and injectable formulations. Since it is widely used in pediatric and geriatric patients, it has been widely dispensed as extemporaneously compounded suspensions. Stability data to calculate the shelf life for such a formulation at any temperature have not been reported. However, stability data for extemporaneously compounded formulations at room temperature and under refrigeration have been previously reported.4,5 The purpose of this study was to improve upon previous extemporaneous formulations of amiodarone hydrochloride and to study its stability at various temperature conditions as per International Conference of Harmonization guidelines. Characterization of the degradation products was also undertaken using high-performance liquid chromatographic-mass spectrometry (HPLC-MS).
Methods
Preparation of Amiodarone Hydrochloride Suspension
Conclusions
This study achieved three goals. The extemporaneously compounded formulation provided a reasonably palatable amiodarone hydrochloride formulation that can be used for both pediatric and geriatric patients. The formulation extended the shelf life that was previously reported by Nahata et al.7 The shelf life was calculated to be 193.42 36 days at 25 deg C. At 4 deg C, the shelf life was found to be 677.26 days. A mechanism has been proposed that can explain the presence of degradation products. The proof for this proposed mechanism is left to the medicinal chemists to ponder.
Acknowledgment
The authors wish to acknowledge the generous contribution of Pacerone tablets by Upsher Smith, Minneapolis, Minnesota, which facilitated the timely progression of this project.
References
1. Florey K. Analytical Profiles of Drug Substances. New York:Academic Press, Inc.; 1991;20:1-88.
2. Nuwer MR, Browne TR, Dodson WE et al. Generic substitutions for antiepilectic drugs. Neurology 1990;40:1647-1651.
3. Kuga K, Yamaguchi I, Sugishita Y. Effect of intravenous amiodarone on electrophysiologic variables and on the modes of termination of atrioventricular reciprocating tachycardia in Wolff-Parkinson-White syndrome. Jpn Circ J 1999;63:189-195.
4. Nahata MC. Stability of amiodarone in an oral suspension stored under refrigeration and at room temperature. Ann Pharmacother 1997;31:851-852.
5. Nahata MC, Morosco RS, Hipple TF. Stability of amiodarone in extemporaneous oral suspensions prepared from commercially available vehicles. J Ped Pharm Pract 1999;4:186-189.
6. Lachman L, Deluca P, Akers M. Kinetic principles and stability testing. In: Lachman L, Lieberman HA, Kanig JL, eds. The Theory and Practice of Industrial Pharmacy. Philadelphia:Lea & Febiger; 1972:671-673.
7. Nahata MC, Hipple TF Pediatric Drug Formulations. 2nd ed. Cincinnati:Harvey Whitney Books; 1992:25.
Address correspondence to: Kenneth S. Alexander, PhD, RPh, The College of Pharmacy, The University of Toledo, Toledo, OH 43606. E-mail: kalexan@utnet.utoledo.edu
Kenneth S. Alexander, PhD, RPh
N. Thyagarajapuram
College of Pharmacy
The University of Toledo
Toledo, Ohio
Copyright International Journal of Pharmaceutical Compounding Sep/Oct 2003
Provided by ProQuest Information and Learning Company. All rights Reserved