During six consecutive months, seven patients admitted to our ICU (15 beds, general ICU, approximately 300 intubated patients per year) for acute respiratory failure requiring intubation and mechanical ventilation presented with a peculiar neuromuscular disorder. After the occurrence of this cluster group of patients, we detected two more similar but isolated cases in the following 18 months, ie, altogether 9 patients in 2 years of observation, or 1.55 percent of all intubated patients in our ICU. Sedation was achieved using midazolam, curarization was effected with the neuromuscular non-depolarizing agent pancuronium bromide (PB), and corticosteroids were administered to eight patients. Shortly after discontinuation of sedation and curarization, we observed a ersistent tetraparetic syndrome and/or peroneal palsy with a concomitant increase of serum creatine kinase (CK). None of the patients was septic or had the multisystem organ failure. A strong association between CK increase and PB administration was found, whereas no patient suffered severe liver or kidney failure. The duration of the neurologic deficit ranged from 4 to 52 weeks, with only partial recovery for some patients; the duration of dysfunction was apparently related to the total dose of corticosteroids received. Two patients had difficulty being weaned from the respirator and required tracheostomy. Electrophysiologic studies showed signs of axonal neuropathy and myopathic changes, ie, motor units of brief duration, small amplitude, overly abundant for the voluntary effort being exerted. Muscle biopsies showed significant myopathic alterations, with foci of muscle necrosis in most patients and minimal lymphocytic inflammation in one patient. The neurologic complication described differs from the polyneuropathy in critically ill patients. Furthermore, PB or corticosteroids or both appear to be the causal agents. The duration of the neuromuscular dysfunction may be related to concomitant steroid therapy. The CK enzyme seems to be a marker of the disorder. This disorder is associated with myopathic alterations and axonal degeneration in some patients. Pancuronium bromide should be used with caution, particularly when associated with steroids therapy, and it may cause difficulty in weaning patients from the respirator. (Chest 1994; 106:210-20)
CIP=critical illness polyneuropathy;
CK=creatine kinase;
PB=pancuronium bromide
Key words: curare; myopathy; neuropathy; pancuronium bromide; peroneal palsy; quadriparesis
Neuromuscular junction blocking agents are frequently used in intubated mechanically ventilated patients in ICUs. Sometimes, prolonged use of these agents is required, as in asthmatic or ARDS adult respiratory distress syndrome patients.(1) In both adults and children, a clinical picture characterized by prolonged paralysis following discontinuation of the mechanical ventilation and paralytic therapy,(2)(3)(4) particularly in patients having received high doses of corticosteroids,(2) has been recognized. Some of these patients are difficult to wean from the mechanical respirator due to respiratory muscle weakness.(5) Electrophysiologic studies and peripheral muscle biopsies have disclosed variable results in these patients, including neurogenic or myopathic abnormalities associated with some degree of necrosis.(2)(6)
We describe nine patients treated with pancuronium bromide (PB) and midazolam for artificial ventilation, who developed tetraparesis or peripheral nerve palsies or both in the absence of sensory disturbance. We discuss the role of muscle-relaxing drugs and corticosteroids in this neuromuscular disorder.
MATERIALS AND METHODS
We observed seven patients, between October 1989 and March 1990, who manifested an unexplained peripheral neurologic dysfunction. All patients had been intubated, ventilated, and treated with PB and several other drugs. Two additional cases were detected in our ICU in July and December 1991. Anthropometric, clinical, laboratory, and therapeutic data are summarized in Tables 1 through 3.
[TABULAR DATA OMITTED]
Table 3--Drugs Administered (Other Than Pancuronium Bromide)
(*)Drugs potentially neurotoxic and/or myotoxic, as noted by Goodman et al.(51)
We performed a systematic retrospective review of all patients admitted to our ICU between January 1, 1982, and October 1, 1989, to disclose similar cases in our institution (university hospital, 1,600 beds; 15 medical ICU beds, 250 to 300 intubated patients per year).
As shown in Tables 1 through 3, all patients were mechanically ventilated. Their clinical diagnoses differed, as did duration of mechanical ventilation and doses of PB and of other drugs administered (Table 4). Pancuronium bromide was the only agent consistently found to be associated with repeated total creatine kinase (CK) elevation. The details of the patients' histories are described in the following section. Intubation day is counted as day 0.
[TABULAR DATA OMITTED]
CASE REPORTS
CASE 1
A 69-year-old woman (cluster case) with COPD was intubated because of respiratory failure due to bronchopulmonary infection. Between days 1 and 4, the patient received PB intravenously (total amount, 120 mg). On day 3, we observed a peak of CK (444 unit/L). A flaccid tetraparesis with absent stretch reflexes was noticed at extubation day. She began to move her arms at day 6 and she had near normal strength on day 9, with reappearance of reflexes. After 1 month, recovery was complete.
CASE 2
A 68-year-old woman (cluster case) was intubated because of bilateral pneumonia. Between days 1 and 5, the patient received PB intravenously (total amount, 124 mg). On day 2, we observed a peak of CK (6,440 unit/L). After 10 days, a flaccid tetraparesis with absent stretch reflexes was noticed. Two days later, tetraparesis began to improve and the patient could be extubated on day 20. A moderate paresis was still present. She left the hospital on day 42 with a persistent paresis and paresthesia of the 5th finger.
CASE 3
A 40-year-old man (cluster case) was intubated because of encephalitis with coma and seizures. He needed barbiturates for status epilepticus. The first day, the patient received PB intravenously (total amount, 6 mg). On day 2, we observed a peak of CK (4,200 unit/L). The patient's condition improved and he was extubated on day 14. Three days later, a flaccid tetraparesis with absent stretch reflexes was noticed. The condition persisted for 24 days with a slow improvement.
CASE 4
A 57-year-old woman (cluster case) with COPD was intubated because of respiratory failure. Between days 1 and 5, the patient received PB intravenously (total amount, 74 mg). On day 4, we observed a peak of CK (730 unit/L). Five days after extubation, a left peroneal palsy was noticed. On the next day, right peroneal and tibial palsies appeared. Electromyography revealed a more widespread disorder, because fibrillations and positive sharp waves were observed in proximal and distal musculature as well as in musculus spinalis. The patient left the hospital 50 days later with a left peroneal palsy, which was severe enough to cause a stepping gait. Eleven months later, she had completely recovered.
CASE 5
A 63-year-old man (cluster case) was intubated because of an acute asthmatic attack. On day 1, the patient received PB intravenously (total amount, 2 mg). Between days 4 and 9, again received 48 mg of PB, as well as at day 15 (2 mg). After each dose of PB, we observed an increase in CK; the respective values (peaks) were 550, 1,650, and 1,250 unit/L. Shortly after extubation, a flaccid tetraparesis with absent reflexes was noticed. Three days later, the patient was reintubated because of respiratory muscle fatigue. He could be extubated again 4 days later. The paresis was then improving and the reflexes were again present, albeit weaker. On day 22 after hospitalization, the muscle strength was near normal, but a right peroneal palsy was noticed. The patient left the hospital with a stepping gait that resolved 1 year later.
CASE 6
A 76-year-old man (cluster case) was intubated because of bilateral pneumonia with septic shock. Between days 1 and 6, the patient received PB intravenously (total amount, 192 mg), and on day 2, we observed a peak of CK of 1,520 unit/L. On day 6, weaning from the respirator was attempted, but the patient was unable to trigger the ventilator. A day later, a flaccid tetraparesis with absent reflexes was noticed. Between days 7 and 12, PB was again prescribed due to difficulties in performing mechanical ventilation; once again, a peak of CK (1,320 unit/L on day 11) was noticed. After 19 days, the patient's inability to be weaned from the respirator necessitated a tracheostomy, which was closed after 25 days. The neurologic condition improved slowly and the patient could leave the hospital after 123 days with a complete recovery.
CASE 7
A 72-year-old man (cluster case) with COPD was intubated because of respiratory failure. Between days 1 and 3, the patient received PB intravenously (total amount, 32 mg), and on day 2, we observed a peak of CK of 6,100 unit/L. After 4 days, weaning from the respirator was attempted, but it was found to be impossible due to respiratory muscle fatigue. Due to difficulties in performing mechanical ventilation, PB again was prescribed between days 6 and 11 (total dose, 34 mg), and on day 7, a new peak of CK (1,100 unit/L) appeared. A moderate tetraparesis with diminished reflexes was noticed at day 12. After 19 days, a tracheostomy was performed, and on day 29, weaning was achieved. During the next week, the paresis improved and the reflexes reappeared, with a persistent peroneal palsy. On day 74, the patient left the hospital with a slight tetraparesis and a left peroneal palsy, causing a stepping gait. The general weakness disappeared over the next weeks, but the peroneal palsy persisted for a year.
CASE 8
A 77-year-old woman with COPD was intubated because of respiratory failure. The first day, the patient received PB intravenously (total amount, 8 mg), and we observed a peak of CK on day 2 (550 unit/L). Between days 10 and 13, 17 mg of PB was prescribed, and a new peak of CK was present (1,480 unit/L). On day 16, a flaccid tetraparesis with preserved stretch reflexes was noticed. This condition persisted throughout the hospital stay, with a slow improvement and the patient left the hospital on day 140 with a near complete recovery, which was achieved 1 month later.
CASE 9
A 57-year-old man was intubated due to an acute asthmatic attack. Between days 1 and 2, the patient received PB intravenously (total amount, 52 mg), and at day 3, we observed a peak of CK (990 unit/L). Twelve milligrams of PB was again prescribed for a transient recurrence of asthma at day 4, and on the day after, a peak of CK (4,980 unit/L) was observed. On day 6, a flaccid tetraparesis with diminished reflexes was noticed. On day 24, the patient left the hospital with a slight residual paresis, which disappeared over the next weeks.
ELECTROPHYSIOLOGIC FINDINGS
Electrophysiologic studies were carried out in the ICU using surface-stimulating and recording electrodes, according to standard techniques. The sensory studies were usually antidromic. Each time when the amplitude of the response was abnormally small, orthodromic measurement using near nerve recording was performed. High voltage (up to 100 V) long duration (up to 1 ms) electrical stimuli were used when apparently inexcitable motor nerves were being tested. A search for conduction blocks was performed in every patient by stimulating at different levels along the nerves, using criteria described previously.(7) Neuromuscular transmission was assessed in seven patients by repetitive 3-, 20-, and 30-Hz stimulations of the ulnar nerve with recording from musculus abductor digiti minimi manus. Electromyographic concentric needle electrode recordings were obtained from several muscles. Control tests were performed a few days or weeks later in five patients.
PATHOLOGIC STUDIES
Biopsies of the musculus quadriceps femoris were obtained from seven patients, whereas one patient or the patient's family refused the procedure (patients 1 and 2). The muscle specimens were frozen in isopentane, cooled with liquid nitrogen at -170[degrees]C, and stored at -70[degrees]C. Frozen sections were cut at 6 [micro] GM 13 and stained routinely with hematoxylin and eosin, modified Gomori, van Gieson-elastin, oil red O, and periodic acid-Schiff. Histochemical techniques for adenosinetriphosphatase (with preincubation at pH 9.4, 4.6, and 4.3) and nicotinamide dinucleotidetetrozolium reductase (NADH-TR) were also performed, as well as acid phosphatase and succinic dehydrogenase. Immunohistochemical techniques using leukocyte common antigen by the avidine-biotine complex (ABC) method was used to better characterize any inflammatory component. Small sections of fresh muscle also were fixed in 2 percent glutaraldehyde solution in cacodylate buffer. Blocks from these were embedded in epoxy resin, and semithin sections were prepared and stained with toluidine blue. From selected areas, ultrathin sections were obtained and examined using a Phillips 400 electron microscope.
ENVIRONMENTAL INJURY
Because we observed a cluster group of seven patients during a short period, ie, between October 1989 and April 1990, we tried to detect some modification in our ICU environment: no changes in the technology (same respirators, tubing, filters, cleaning substances, sterilization procedures) or in the architecture and climatization in the unit could be found during this period. Similarly, we were unable to detect any modification in our therapeutic habits and in the nurses' care procedures. Finally, we attempted to determine if the numerous drugs prescribed in these patients, particularly PB, midazolam, and methylprednisolone, were manufactured at a close time by the pharmaceutical companies, and could have contained some "toxic" component; these drugs were not issued from common lots or packages or both. In addition, between April 1990 and December 1991, whereas the incidence of the neuromyopathic syndrome dropped from 14 cases per year to 1.2 case per year, nothing had apparently changed in our ICU.
RESULTS
Clinical and Laboratory Data
In all patients, we measured the following blood levels: thyroid function tests (thyroid stimulating hormone, triiodothyronine uptake and tetraiodothyronine), albumin and transferrin, and blood electrolytes (sodium, potassium, phosphate, calcium, and magnesium). The measurements were performed at admission and then at regular intervals during the hospitalization; none of these levels became abnormal at any time.
The total serum CK value was elevated in all patients, and as mentioned in the Case Reports section, this elevation was clearly related (in time) to PB administration. As a mean, our patients showed 2.1 (range, 1 to 4) CK peaks, ie, altogether 19 peaks were observed for all patients during the period of observation, whereas PB was administered immediately prior to 15 (79 percent) of these CK peaks. The CK-MB subunit never exceeded 3 percent of the total CK value; therefore, it will not be reported in our further discussion and analysis. When considering the averaged highest CK peak of each patient, the CK values were 3,179 [+ or -] 2,514 (SD) unit/L, with a median of 1,780 unit/L and a range of 450 to 6,800 unit/L. We also computed the surface under the curve of CK for the whole period of observation (value above the normal range of 150 unit/L at our institution) vs time for all patients: the mean [+ or -] SD was 15,805 [+ or -] 13,304 unit/L X days, whereas the median was 13,963 (range, 1,672 to 44,451).
For all patients, there was no relationship between the total PB dose given and (1) the highest peak's CK value (r=0.299; p=0.755) and (2) the surface of CK under the curve vs time (r=0.530; p=0.372). Similarly, no correlation could be found between the total PB dose administered or the total PB treatment duration and the severity or the duration of neurologic dysfunction (r=0.478; p=0.459 and r=0.589; p=0.278, respectively).
In addition, no relationship was detected between peak CK value and (1) the duration of mechanical ventilation (r=0.328; p=0.711) and (2) the duration of neurologic symptoms (r=0.214; p=0.868). Similarly, the correlations between the total surface under the curve of CK vs time and the same parameters were nonsignificant (r=0.442; p=0.521 and r=0.324; p=0.716, respectively).
Furthermore, we pooled all CK peaks for all patients, ie, 19 peaks, for statistical analysis. As for previous statistics, there was no correlation between the PB dose administered just prior before a given peak and the absolute CK value of this particular peak (r=0.128; p=0.876) or the surface under the curve for CK of this same peak (r=0.129; p=0.874).
A multivariate analysis, considering the duration of neurologic symptoms (weeks) as the dependent variable and six parameters (age of the patients, total PB dose, PB treatment duration, peak total CK, and total CK area under the curve total corticosteroid dose) as co-independent parameters, was performed. The partial correlation coefficients were r=0.262 for the age; 0.361 for total PB dose; 0.469 for PB treatment duration; and 0.071 for peak total CK; 0.124 for CK surface; and 0.696 for total corticosteroid dose. This latter only can be considered as statistically significant (p=0.037).
Electrophysiologic Study
Electrodiagnostic data are summarized in Table 4. Sensory nerve action potentials were either normal, of reduced amplitude (patients 2, 6, 8, and 9), or absent (left ulnar nerve in patient 2, both superficial peroneal nerves in patient 4, left superficial peroneal nerve in patient 7). Upper limb sensory conduction velocities were reduced in patients 2, 6, 7, 8, and 9. Lower limb sensory conduction velocities, tested in 7 patients, were reduced in patients 4, 6, 7, and 8. Compound muscle action potentials were markedly diminished in amplitude in most patients at first examination; this was much improved in the control examinations. Motor conduction velocities were within normal range or slightly reduced in patient 8. There were no conduction blocks detected. Neuromuscular transmission, as assessed by repetitive stimulation, was normal in all seven patients tested. Fibrillation potentials and positive sharp waves were found in most distal as well as proximal muscles; they were found in the musculus spinalis in all seven patients tested. Fibrillations and positive sharp waves were markedly less abundant in subsequent examinations, sometimes as early as a few days later in patients 2 and 8; they persisted for a longer period of time in muscles innervated by the nerves that showed signs of marked axonal degeneration, as attested by sensory nerve action potentials of low amplitude (peroneal nerve in patient 9) or by the absence of such (ulnar nerve in patient 2, peroneal nerves in patients 4 and 7). In five patients, volitional activity could not be studied during initial examination due to sedation or to the severity of the weakness. In spite of this, a myopathic pattern consisting of motor units of brief duration, small amplitude, overly abundant for the amount of power being exerted on slight voluntary effort, was observed in patients 4 and 8 during the initial examination, and in patients 2 and 7 during subsequent control studies.
Pathologic Findings
Edema was present in what could be considered the acute, early (patients 7 and 9) or subacute (patient 4) phases, accompanied by focal necrosis (isolated or small groups of fibers). All biopsy specimens showed degenerative changes of variable degrees, as well as evidence of regeneration characterized by the presence of small fibers with a basophilic cytoplasm and vesicular nuclei (Fig 1). Ring fibers were present in most of the cases, but fiber splitting as well as targetoid fibers were only occasionally observed. Internal nuclei also were present. Fat droplets were increased mainly in fibers undergoing necrosis or degenerative changes. In one case (patient 4), aggregates of lymphocytes surrounded some fibers in necrosis. Atrophic polygonal fibers were always present, either perifascicular or intrafascicular or both, and in all cases, some of these were angulated, principally the type 2 fibers (Fig 2). In most cases, type 1 fibers were the most severely damaged, resulting in some degree of fiber type grouping and type 2 fiber predominance, with the exception of two cases (patients 4 and 8) in which there was type 1 fiber predominance in some areas. Fibrosis was inconspicuous, except in two cases, one of which (patient 8) had areas of fatty infiltration which was surely unrelated to the actual disease. In the apparently late stages of the condition (patient 6), the degenerative and regenerative changes were minimal, but diffuse, with many groups of atrophic, angulated fibers. The ultrastructural features were quite variable, depending on the duration of treatment, the doses administered, and the time the biopsies were done. In the acute phases, there was extensive myofibrinolysis, especially of the thick myofilaments. The A band loss and disorganization of the myofilaments were often associated with groups of nemaline rodlets and Z band streaming (Fig 3). Mitochondria were somewhat enlarged and irregular and some of them presented with myelin bodies. Dilatation of T tubules was sometimes present, taking on a beehive appearance. In later stages, these abnormalities were less frequent (Fig 3). Three cases showed filamentous bodies. Table 5 summarizes the pathologic findings.
[CHART OMITTED]
DISCUSSION
The main findings of this series are as follows: (1) A clinical syndrome characterized by an acute generalized neuromuscular weakness consistently following PB administration, with increased serologic skeletal muscular enzymes. (2) An electrophysiologic pattern of low amplitude motor-evoked potentials with diffuse spontaneous electromyographic activity (fibrillations and positive sharp waves), myopathic changes, and signs of focal axonal degeneration in some patients. (3) Morphologic changes suggestive of mixed neuromyopathic pathologic characteristics. (4) A possible interaction between PB and corticosteroid treatment in the development and duration of the neuromuscular disorder. (5) The intriguing feature of a cluster group of patients, suggesting some as yet undefined factor combined with a drug effect as probably responsible for the syndrome.
Neuromuscular dysfunction in ICU patients is well recognized in the literature. It appears to be rather frequent in critically ill patients, particularly in septic syndromes, multisystem organ failure, and prolonged stay in ICU for severe diseases.(8)(9)(10) The most frequently described neurologic problem often is referred to as the critical illness polyneuropathy (CIP). Although our patients required mechanical ventilation and were typical acutely ill ICU patients, we do not think that the disorder that they presented corresponds to CIP as described by many authors.(11)(12)(13)(14)(15)(16) First, none of our patients was severely septic, hemodynamically unstable,(8)(9)(10) or featured the multisystem organ failure, as consistently reported in the CIP; only patients 2 and 8 suffered from a bacteriemic pneumococcal episode, but these infections were of short duration, easily controlled by antibiotics, and were not associated with any characteristics of the sepsis syndrome.(17) Second, despite the fact that patients 3, 8, and 9 had mild renal dysfunction, our other patients had no organ failure and, therefore, the criteria for multisystem organ failure(10) were never fulfilled. Furthermore, the CIP consists of a diffuse sensory and motor axonal polyneuropathy, whereas the disorder encountered in our patients consisted mainly of a motor deficit, and outcome was good, except for persistence of paresis of the peroneal innervated muscles. Finally, myopathic changes are not a feature of CIP, whereas they existed in all of our patients. In our opinion, the two disorders differ in many ways.
Because we observed primarily an increase in cytolytic skeletal muscle enzymes, ie, CK, aspartate aminotransferase, and aldolase, associated at histologic examination with necrosis and focal atrophic fibers, with some regeneration, one could discuss the catabolic myopathy syndrome.(14)(18) However, this condition is, like CIP, clearly asociated with the sepsis syndrome, multisystem organ failure, and very protracted residence in ICU under difficult conditions; none of our patients featured these characteristics.
The absence of albuminocytologic dissociation in the CSF and of nervous conduction blocks are strong arguments against the diagnosis of a Guillain-Barre polyradiculoneuritis, even in an acute axonal form.(19) Finally, there was no electrophysiologic feature of myasthenia gravis, a disease that may be precipitated by PB and several other drugs.(20)
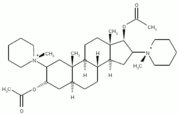
Our patients more likely manifest a distinctive neuromuscular syndrome related to some other cause. Drug administration, particularly muscle relaxants and corticosteroids, is suspected, a possibility which has already been envisaged by others,(2)(21)(22)(23)(24)(25)(26) and which fits with our data. Pancuronium bromide is a biquarternary aminosteroid with two acetylcholine-like fragments responsible for neuromuscular blockade. This compound acts via a postsynaptic blockade of the acetylcholine receptors and to a lesser extent by presynaptic inhibition of the neurotransmitter. The drug is rapidly eliminated by the liver and the kidneys.(27) Therefore, patients with liver or renal failure may present a prolonged neuromuscular blockade when treated with PB,(28) as well as myasthenia gravis patients,(29) and patients treated with several other drugs. Among the latter, electrolytes (lithium, magnesium) and some antibiotics have been implicated in prolonging the action of PB, especially polymyxin B(30) and aminoglucosides.(31) The interaction between PB and corticosteroids appears to be complex; PB is a compound presenting a steroid nucleus. A reversal of PB-induced neuromuscular blockade has been reported to occur during the intravenous administration of hydrocortisone(32) or massive doses of prednisone.(33) On the other hand, other nondepolarizing blocking agents, like vecuronium, may cause severe muscle weakness with early muscle wasting, when associated with corticosteroids, a phenomenon apparently similar to the one observed in our series.(26) Several groups have described patients who may have presented similar problems, particularly asthmatic subjects or other patients with an acute respiratory failure, submitted to intubation, mechanical ventilation, sedation, and paralysis with nondepolarizing blocking agents and corticosteroids.(2)(5)(13)(34)(35) In our patients, there was a clear timely relationship between PB administration, the clinical weakness, and the skeletal muscle injury, assessed by the plasma CK increase. In addition, some degree of association with the total dose of corticosteroid and the duration of the neuromuscular symptoms was found. The other drugs seemed not to have played a role in the neurologic dysfunction (Table 3).
Steroid myopathy also should be considered; it usually develops when patients are treated for a prolonged period, particularly with fluorinated compounds.(35) This complication may also occur with prednisone, prednisolone, dexamethasone, and methylprednisolone,(36) even when used for short periods.(24) Characteristically, however, and contrary to the observations made in our patients, serum muscular enzyme plasma levels (CK, aldolase, etc.) were normal.(36) In corticosteroid myopathy(36)(37) or Cushing syndrome,(38) the electromyogram shows myopathic changes but no spontaneous activity on needle electrode examination, whereas it was a characteristic feature in our patients. In steroid myopathy, symmetrical weakness involves the proximal limb muscles and spares the respiratory musculature,(35)(36)(39) whereas weakness was generalized in our subjects. In fact, one finds no convincing evidence in the literature of an acute steroid myopathy that occurred without the administration of other drugs, particularly neuromuscular blocking agents.(5)(13)(24) Finally, patient 2, and several patients from J. C. Couturier, M.D. (personal communication, 1993) did not receive any steroids. For all these reasons, we believe that PB was the main cause for the acute neuromuscular disorder which we observed, although steroids have probably played some role, as shown by the correlation between the steroid total dose and the duration of the neurologic dysfunction, an observation consistent with the data of others.(36)(37)(40)
The electrophysiologic study reveals a particular pattern. The findings of the electroneurography, ie, low amplitude compound muscle action potentials with preserved conduction velocities, may be encountered in motor axonal neuropathies, in disorders of the neuromuscular junction, or in myopathies. Repetitive nervous stimulation disclosed no evidence of myasthenia gravis or of myasthenic syndrome. Needle electromyography revealed a profuse spontaneous activity. This latter, although most commonly seen in case of axonal degeneration, is not specific; it also may be encountered in primary muscle disorders, severe neuromuscular junction dysfunction, and in some metabolic disorders. With respect to the activity recorded in the peroneal innervated muscles in some of our patients (patients 4, 7, and 9), the spontaneous activity differed somewhat with that seen in the case of axonal degeneration: (1) this activity often was very abundant early after tetraparesis was discovered, whereas it is usually first observed 2 to 3 weeks after axonal lesions; (2) this activity diminished rapidly, and clinical recovery often was complete in a few weeks. In axonal neuropathies, denervation potentials tend to persist for months and outcome usually is not favorable so rapidly. Franc et al,(41) in serial electromyographic studies of patients who were ventilated and underwent ventilation, curarization, have shown that fibrillations and positive sharp waves exist early and disappear about 40 min to 12 days after curarization was discontinued. These authors suggested that this pattern, reminiscent of a denervation pattern, was related to curarization and could be considered as artifactual or functional. This constitutes another clue pointing to the role of PB in the observations made in our patients. In the few subjects in whom voluntary activity could be obtained, there were electromyographic signs of myopathic changes, a finding consistent with the elevated CK and the alterations seen in the muscle biopsies. Signs of myopathy on the electromyogram with spontaneous potentials (fibrillations, positive sharp waves and repetitive discharges) observed diffusely and particularly in the musculus spinalis is a common finding in myositis, but muscle biopsies revealed no inflammation, or only a few lymphocytes in one case (patient 4). Finally, mononeuropathic axonal degeneration of peroneal nerves were present, as shown by the abnormal sensorimotor neurography and the protracted clinical paralysis in some patients. Neurographic examination disclosed no evidence of compression at the fibular head, that is, no conduction block or focal slowing. Our ICU nursing conditions are such that no peroneal palsy was observed in other patients during the last 5 years (about 8,000 patients, among them about 1,500 were intubated).
Histologic examination of our cases was somewhat different from the published data recorded in patients suffering from the CIP; fiber necrosis, with some regenerative foci were frequently observed in our series, as well as intrasarcoplasmic vacuolizations and moth-eaten appearances. These latter have not been described to date in the CIP.(11)(12)(13) We also compared the histologic features recorded in our patients with the data published by others on the myopathy associated with drugs, particularly PB and corticosteroids.(2)(21)(24) In our patients, a severe muscular necrosis, with regenerative aspects, was more frequent in the early phases of the condition than it was described in curare-associated myopathy. In addition, some characteristics indirectly suggested the occurrence of neurogenic myopathic changes, with very little inflammation. These features are tent with the electrophysiologic data.
The pathogenic mechanisms of the neuromuscular disorder that we have observed are poorly understood. Depolarizing neuromuscular blocking agents are known to induce fasciculations and myalgias(42) and, sometimes, plasma CK and urine myoglobin elevation and renal failure.(43)(44)(45) However, nondepolarizing agents, such as PB, are not known to cause such disturbances, and they are advocated as a pretreatment to prevent suxamethonium- or succinylcholine-induced myalgia and rhabdomyolysis.(44)(46)(47) In neonates, skeletal muscle growth failure and neuromyopathic changes due to PB or other nondepolarizing blocking agents have only been described with prolonged use of these agents.(3)(4) An acute quadriplegia that is reversible in a few weeks must be related to some functional disorder such as (1) nervous conduction blocks, as for instance in the Guillain-Barre syndrome; these were thoroughly searched for but not found, and normal cerebrospinal fluid ruled out this diagnosis; (2) neuromuscular junctional blockade; (3) metabolic disorders affecting membrane properties as for instance saxitoxin. There were no clinical clues in such directions. Of these possibilities, a persistent neuromuscular blockade is the most appealing hypothesis, since the role of PB could be strongly suspected. Surprisingly, repetitive nerve stimulation revealed no gross abnormality. Finally, we have no explanation for the occurrence of the neuromuscular disorder in some patients but not in others. The systematic retrospective review of all patients admitted to our ICU between January 1982 and October 1989 (12,345 patients; 2,542 intubations) disclosed no similar case. An anecdotal case of J. C. Couturier, M.D. (personal communication, 1993) suggested some form of idiosynchrasy: an asthmatic patient presented the complication twice when he was again exposed to PB and corticosteroids for the recurrence of an acute asthmatic attack. The role of some intercurrent unknown factor is suggested by the cluster group of patients in this series. However, our environmental study disclosed no such evidence.
Finally, although the mechanism of this likely drug-induced disorder remains uncertain, the severe complications that the patients may suffer prompt us and others(2) to propose some prophylactic principles: PB should be prescribed at the lowest dosage possible,(48) for a short time, in such a way that only partial curarization is achieved. Monitoring of neuromuscular transmission, leaving myotatic reflex intact, should be used. Combined treatment of PB with aminoglycosides or other drugs potentially interfering with neuromuscular transmission should be avoided. Patients receiving PB treatment with poor renal or liver function should be monitored with extra alertness,(28) whereas several risk factors for developing the syndrome begin to be identified and should be avoided: acidemia, hypermagnesemia, accumulation of the 3-desacetyl- and 3-hydroxy-metabolites of PB (and vecuronium).(49)(50) Due to possible idiosyncrasy, special caution should be exerted with patients who have presented the complication during a previous hospitalization.
In conclusion, we describe nine ICU patients presenting with a neurologic complication that clearly differs from other neuromuscular dysfunctions observed in ICU patients, such as the CIP or the catabolic myopathic syndrome. The disorder observed in our patients is characterized by an acute generalized weakness, first noticed when curarization is discontinued. Course is variable with possible relation to the dose of corticosteroids administered. Recovery is usual but residual peroneal palsy is frequent. The CK enzyme kinetic seems to be a biological marker of the syndrome, since an increase was consistently associated with PB administration in all nine patients. This enabled us to strongly suspect PB to be the causal agent of the disorder. Electrophysiology shows motor-evoked responses of reduced amplitude as well as signs of myopathic changes. A typical electromyographic pattern consists in the early appearance, and often early disappearance, of a profuse spontaneous activity, present in most muscles (including spinalis). Muscle biopsies show variable degree of degenerative changes and of focal myonecrosis in all patients. No convincing explanation was discovered for the temporal clustering of seven of our nine patients. In mechanically ventilated patients, PB should be used with caution, particularly when corticosteroid administration also is involved.
ACKNOWLEDGMENTS: We thank Dr. J.C. Couturier for useful suggestions and comments, and Dr. P. Jolliet for criticism and help in the preparation of the manuscript. Our thanks also to Ms. J. Stalder and Mr. T. Le Minh for technical assistance, to Mr J.C. Rumbeli for the photographs, and to Professor T. A. Seemayer for careful review of the manuscript.
REFERENCES
(1)Gurevitch M, Van Dyke J, Young E. Improved oxygenation and lower peak airway pressure in severe adult respiratory distress syndrome. Chest 1986; 89:211-13
(2)Op de Coul A. Lambregts P, Koeman J, van Puyenbroek M, Ter Lak H, Gabreels-Festen A. Neuromuscular complications in patients given Pavulon[R] (pancuronium bromide) during artificial ventilation. Clin Neurol Neurosurg 1985; 87:17-22
(3)Rutledge M, Hawkins E. Skeletal muscle growth failure induced in premature newborn infants by prolonged pancuronium treatment. J Pediatr 1986; 109:883-86
(4)Torres C, Maniscalco W, Agostenelli T. Muscle weakness and atrophy following prolonged paralysis with pancuronium bromide in neonates. Ann Neurol 1985; 18:403-09
(5)Picado C, Montserrat J, Agusti-Vidal A. Muscle atrophy in severe exacerbation of asthma requiring mechanical ventilation. Respiration 1988; 53:201-03
(6)Gooch J, Suchyta M, Balbierz J, Petajan J, Chemmer T. Prolonged paralysis after treatment with neuromuscular junction blocking agents. Crit Care Med 1991; 19:1125-31
(7)Magistris MR. Les blocs de conduction nerveuse. In: Cadilhac J, Dapres G, eds. EMG: actualites en electromyographie. Sauramps Med 1991; 123-38
(8)Witt N, Zochodne D, Bolton C, Grand'Maison F, Wells G, Bryan Young G, et al. Peripheral nerve dysfunction in sepsis and multiple organ failure. Chest 1991; 99:176-84
(9)Baue A. Multiple, progressive, or sequential system failure: a syndrome of the 1970s. Arch Surg 1975; 10:779-81
(10)Carrico D. Multiple system organ failure. Surg Clin N Am 1988; 68:107-22
(11)Bolton C, Laverty D, Brown J, Witt N, Hahn A, Sibbald W. Critically ill polyneuropathy: electrophysiological studies and differentiation from Guillain-Barre syndrome. J Neurol Neurosurg Psychiatr 1986; 49:563-73
(12)Bolton C, Gilbert J, Hahn A, Sibbald W. Polyneuropathy in critically ill patients. J Neurol Neurosurg Psychiatr 1984; 47:1223-31
(13)Bolton C, Brown J, Sibbald W. The electrophysiologic investigation of respiratory paralysis in critically ill patients. Neurology 1983; 33:186-92
(14)Zochodne D, Bolton C, Wells G, Gilbert J, Hahn A, Brown J. Critical illness polyneuropathy: a complication of sepsis and multiple organ failure. Brain 1987; 110:819-42
(15)Barat M, Brochet B, Vital C, Mazaux J, Arne L. Polyneuropathies au cours de sejours prolonges en reanimation. Rev Neurol 1987; 143:823-31
(16)Lopez Messa J, Garcia A. Acute polyneuropathy in critically ill patients. Intensive Care Med 1990; 16:159-62
(17)Bone R, Fisher C, Clemmer T, Slotman G, Metz C, Balk R. The methylprednisolone severe sepsis study group: sepsis syndrome, a valid clinical entity. Crit Care Med 1988; 17:389-93
(18)Bolton C. Polyneuropathy as a cause of respiratory failure in critical illness. Intensive Care Dig 1988; 7:7-9
(19)Feasby T, Gilbert J, Brown W. An acute axonal form of Guillain-Barre polyneuropathy. Brain 1986; 109:1115-26
(20)Belafsky M, Klawans H Jr. Prolonged neuromusculkar blockade with pancuronium bromide in a young healthy woman. Anesthesiology 1974; 40:295-96
(21)Van Marle W, Woods K. Acute hydrocortisone myopathy. Br Med J 1980; 281:271-72
(22)Laflin M. Interaction of pancuronium and corticosteroids. Anesthesiology 1977; 47:471-72
(23)Noirot A, Floriot C, Daoudal P, Delacour J, Wagschal G. Myopathie au decours d'une curarisation prolongee. Rean Soins Intens Med Urg 1990; 6:186
(24)Hirano M, Ott B, Raps E, Lennihan, Hair L, Hays A. Acute steroid myopathy and nondepolarizing blocking agents [Abstract]. Neurology 1991; 41(suppl 1):1064 P
(25)Danon M, Carpenter S. Myopathy with thick filament (myosin) loss following prolonged paralysis with vecuronium during steroid treatment. Muscle Nerve 1991; 14:1131-39
(26)Margolis B, Khachikian D, Friedman Y, Garrard C. Prolonged reversible quadriparesis in mechanically ventilated patients who received long-term infusions of vecuronium. Chest 1991; 100:877-78
(27)Roizen M, Feeley T. Pancuronium bromide. Arch Intern Med 1978; 88:64-68
(28)Abrams R, Hornbein H. Inability to reverse pancuronium bromide blockade in a patient with renal failure and hepatic disease. Anesthesiology 1975; 42:362-64
(29)Blitt C, Wright W, Peat J. Pancuronium and the patient with myasthenia gravis. Anesthesiology 1975; 42:624-25
(30)Fogdall R, Miller R. Prolongation of a pancuronium-induced neuromuscular blockade by polymyxin B. Anesthesiology 1974; 10:84-87
(31)Martens E, Ansink B. A myasthenia-like syndrome and polyneuropathy: complication of gentamycin therapy. Clin Neurol Neurosurg 1979; 81:241-46
(32)Meyers E. Partial recovery from pancuronium neuromuscular blockade following hydrocortisone administration. Anesthesiology 1977; 46:148-50
(33)McFarlane, I, Rosenthal F. Severe myopathy after status asthmaticus. Lancet 1977; 2:615
(34)Pascucci R. Prolonged weakness after extended mechanical ventilation in a child. Crit Care Med 1990; 18:1181-82
(35)Mastaglia F, Argov M. Drug-induced neuromuscular disorders in man. In: Walton K, ed. Disorders of voluntary muscles. Edinburgh, Scotland: Churchill-Linvingstone, 1981; 873-906
(36)Khaleeli A, Edwards E, Gohil K, McPhail G, Rennie M, Round J. Corticosteroid myopathy: a clinical and pathological study. Clin Endocrinol 1983; 18:155-66
(37)Coomes E. Corticosteroid myopathy. Ann Rheum Dis 1965; 24:465-72
(38)Muller R, Kigelberg E. Myopathy in Cushing's syndrome. J Neurol Neurosurg Psychiatr 1959; 27:314-25
(39)Askari A, Vignos P, Moskowitz R. Steroid myopathy in connective tissue disease. Am J Med 1976; 61:485-91
(40)Golding D, Murray S, Pearce G, Thomson M. Corticosteroid myopathy. Ann Phys Med 1961; 6:161-67
(41)Franc P, Chamelot C, Mahieu P, Knoops P. Interet du suivi electromyographique dans les comas traumatiques ventiles et curarises. In: Les Comas. Paris, France: Societe de Reanimation de Langue Francaise, Flommarion S.A., 1986; 678-85
(42)O'sullivan E, Williams N, Calvey T. Differential effects of neuromuscular blocking agents on suxamethonium-induced fasciculations and myalgias. Br J Anaesth 1988; 60:367-71
(43)Laurence A. Myalgia and biochemical changes following intermittent suxamethonium administration. Anaesthesia 1987; 42:503-10
(44)Blanc V, Vaillancourt G. Succinylcholine, fasciculations and myoglobinaemia. Can Anaesth J 1986; 33:178-84
(45)Hool G, Laurence P, Sivaneswaran N. Acute rhabdomyolytic renal failure due to suxamethonium. Anaesth Intensive Care 1984; 12:360-64
(46)Bennett E, Montgomery S, Dalal F, Prthvi Raj P. Pancuronium and the fasciculations of succinylcholine. Anesth Analg 1973; 52:892-96
(47)Strom J, Jansen E. Pain-reducing effect of self-taming suxamethonium. Acta Anaesthesiol Scand 1984; 28:40-43
(48)Kupfer Y, Mamba T, Kaldawi E, Tessler S. Prolonged weakness after long-term infusion of pancuronium bromide. Am Rev Respir Dis 1992; 117:484-86
(49)Hansen-Flaschen J, Cowen J, Raps E. Neuromuscular blockade in the intensive care unit. Am Rev Respir Dis 1993; 147:234-36
(50)Segredo V, Caldwell J, Matthay M, Sharma M, Grunke L, Miller R. Persistent paralysis in critically ill patients after long term administration of vecuronium. N Engl J Med 1992; 327:524-28
(51)Goodman R, Gilman A, Goodman L, Rall T, Murad F. The pharmacological basis of therapeutics. 7th ed. New York: Mc Millan, 1985; 1066-1239
*From Clinique Medicale I (Dr. Giostra), Division de Neurophysiologie Clinique (Dr. Magistris), Institut de Pathologie Clinique (Drs. Pizzolato and Cox), and Soins Intensifs de Medecine Hopital Cantonal Universitaire, (Dr. Chevrolet) Geneva, Switzerland.
Manuscript received December 15, 1992; revision accepted July 1, 1993.
COPYRIGHT 1994 American College of Chest Physicians
COPYRIGHT 2004 Gale Group