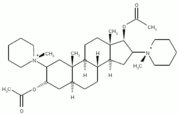
The effects of [±]-2,5-dimethoxy-4-iodoaminophentamine, a serotonin^sub 2A/2C^ receptor agonist, on pharyngeal airflow mechanics were examined in isoflurane-anesthetized lean and obese Zucker rats. The pharyngeal pressure associated with flow limitation, maximum inspiratory flow, oronasal resistance, genioglossus muscle activity, and arterial blood pressure (BP) were measured before and after the intravenous administration of the agonist. A robust activation of the genioglossus muscle in all lean and obese rats was associated with decreased upper airway (UA) collapsibility (p
Keywords: obesity; serotonin; upper airway function
Serotonin (5-HT) plays an important role in the control of upper airway (UA) dilator motoneurons, and it modulates LJA dilator muscle activity across sleep-wake states (1-3). The control of UA motoneurons by 5-HT is thought to be mediated predominantly via 5-HT^sub 2A^ and 5-HT^sub 2C^ receptor subtypes, although other types of receptors may also be implicated (1,4,5). Indeed, intravenous administration of ritanserin, a 5-HT^sub 2A/2C^ antagonist, to awake English bulldogs reduces UA dilator muscle activity and the UA cross-sectional area (6).
The obese Zucker rat (fa/fa), a genetic model of early-onset obesity, has a defective leptin receptor (7) and develops hyperphagia, hyperleptinemia, and hyperinsulinemia (8). These rats exhibit many of the same respiratory deficits as obese humans, including reduced lung volumes, reduced chest wall compliance, blunted ventilatory responses to hypercapnia and hypoxia, and narrowed UA (9, 10). Obese Zucker rats develop morphologic and mechanical changes in respiratory muscle function that are consistent with a chronic overload; the diaphragm becomes weak, and fiber hypertrophy is observed (11).
We have previously reported that the systemic administration of ritanserin had no effect on resting ventilation in older lean Zucker rats but decreased ventilation with a rise in oxygen consumption in older obese Zucker rats (12). This effect of ritanserin on ventilation in older obese Zucker rats was attributed to an increase in UA collapsibility associated with a decline in UA dilator muscle activity. These effects were qualitatively similar to those found when ritanserin was administered to English bulldogs (6), an animal model of sleep-disordered breathing with narrowed UA.
The effects of augmenting UA dilator muscle activity using 5-HT agonists on UA mechanics have not been determined. The consequences of activation of UA dilator muscles on UA pressure-flow relationships using serotonergic agents would be of interest because this knowledge may provide insight on how specific pharmacologie agents may be used to prevent UA collapse. We hypothesized that the administration of a 5-HT^sub 2A/2C^ receptor agonist will increase UA dilator muscle activity and that this increased activity would stabilize the UA. Because the UA is more collapsible in obese Zucker rats compared with lean Zucker rats (12), we also hypothesized that the administration of a 5-HT^sub 2A/2C^ receptor agonist will improve the stability of the UA of obese Zucker rats to levels comparable to those in leans. We examined the effects of the 5-HT^sub 2A/2C^ receptor agonist, [±]-2, 5-dimethoxy-4-iodoaminophentamine (DOI), on pharyngeal airflow mechanics and genioglossus (GG) muscle activity in the isolated UA preparation in lean and age-matched obese Zucker rats. Some of the results of these studies have been previously reported in the form of an abstract (13).
METHODS
A previously described UA preparation (12,14) was used to determine the LJA maximal inspiratory airflow (VImax), the pharyngeal critical pressure (Perit), and oronasal resistance (Ron). Details of the methods are provided in the online supplement. The Institutional Animal Care and Use Committee of the University at Buffalo approved the protocols.
Effects of Serotonin (5-HT)^sub 2A/2C^ Agonist on UA Mechanics (Protocol A)
Eight lean and eight obese Zucker rats were anesthetized, and the femoral vein and artery were cannulated. Electromyogram (EMG) electrodes were implanted into the GG muscle.
The trachea was cut, and an endotracheal tube was placed into the caudal tracheal stub. The animals were mechanically ventilated and continuously anesthetized with isoflurane maintaining end-tidal CO2 at 5%. Five measurements of VImax, Peril, and Ron were obtained 5 minutes after intravenous saline and 5 minutes after DOI (0.5 mg/kg intravenously; Sigma Chemical, St. Louis, MO). Average values were calculated from the five measurements. The magnitude of the changes in UA mechanics induced by DOI was calculated as the delta (Δ).
Effects of 5-HT^sub 2A/2C^ Agonist on UA Mechanics after Neuromuscular Paralysis (Protocol B)
In four lean and four obese Zucker rats, UA mechanics were measured before and after the administration of pancuronium bromide (2 mg/kg intravenously). Fifteen to twenty minutes after paralysis, UA mechanics were measured 5 minutes after the administration of DOI (0.5 mg/kg, intravenously).
Effects of 5-HT^sub 2A/2C^ Agonist on UA Mechanics after Bilateral Hypoglossal Nerve Transection (Protocol C)
Fifteen to 20 minutes after bilateral hypoglossal nerve (cnXII) denervation, UA mechanics were measured 5 minutes after saline and 5 minutes after DOI (0.5 mg/kg, intravenously) in four lean and four obese Zucker rats.
Statistical Analysis
Data were analyzed using SPSS (version 12.0) software (SPSS Inc., Chicago, IL). In protocol A, the differences in UA mechanics, integrated EMG^sub GG^, blood pressure (BP), and heart rate data were analyzed by two-way analysis of variance with repeated measurements on one factor. The between-subject factor was lean versus obese rats. The within-subject factor with repeated measurements was vehicle versus DOI. An interaction term was included. For the paralysis experiments, a similar analysis was employed except the data for lean and obese animals were combined because no differences in response to DOI were seen in the first series of experiments. For the denervation experiments, the effects of DOI were analyzed using paired t test. We compared the systolic and diastolic BP data between the three different protocols using two-way repeated measures analysis of variance (between-subject factor: protocol A vs. protocol B vs. protocol C; within-subject factor: pre-DOI vs. DOI) to determine whether the hypertensive response to DOI was different between the three protocols. All data presented in the text, tables, and figures represent means ± SEM. We checked residuals for outliers and normal distribution. We tested for compound symmetry and made adjustments using the Greenhouse-Geisser correction method when appropriate; p
RESULTS
Effects of 5-HT^sub 2A/2C^ Agonist on Isolated UA Mechanics (Protocol A)
Obese Zucker rats were significantly heavier than lean Zucker rats (p
DOI infusion altered the recruitment profile of the GG muscle. A representative tracing from one lean and one obese Zucker rat is shown in Figure 3. During control (pre-DOI), clear phasic activity in the GG muscle was noted in five of eight lean and in six of eight obese Zucker rats. All rats exhibited low levels of tonic GG muscle activity, as depicted in Figure 3, between the phasic burst activation. After DOI administration, all eight lean and all eight obese Zucker rats exhibited a marked increase in tonic activation of the GG muscle such that no clear phasic activity was detected except in one lean rat. DOI significantly increased the integrated activity of the EMG^sub GG^ (F[1,14] = 14.0, p = 0.002). The integrated activity of the EMG^sub GG^ of lean Zucker rats after DOI was 872 ± 316% of control (p = 0.019) and that of obese Zucker was 878 ± 267% of control (p = 0.019). There was no statistical difference in the integrated EMG^sub GG^ activity between lean and obese Zucker rats after DOI (p = NS).
DOI infusion increased both systolic (F[1, 14] = 70.8, p
Effects of 5-HT^sub 2A/2C^ Agonist on UA Mechanics after Neuromuscular Paralysis (Protocol B)
Eight (four lean and four obese) Zucker rats were studied to explore whether the effects of DOI were neuromuscular in origin and/or indirectly related to the DOI-induced increase in BP. Because the magnitude of the DOI-induced changes in UA mechanics were similar in lean and obese animals in protocol A, we combined the data of the four lean and four obese animals in protocol B. Figure 4 shows the combined mean values of the eight Zucker rats during control condition, after neuromuscular paralysis, and after DOI administration. Complete paralysis with pancuronium resulted in an increase (less negative, more collapsible) Pcrit compared with control (p
Effects of 5-HT^sub 2A/2C^ Agonist on UA Mechanics after Bilateral Hypoglossal Nerve Transection (Protocol C)
To determine the relative conlribution of lhe cnXII in mediating the effects of DOI, we sludied lhe effects of DOI adminislralion in additional eight (four lean and four obese) Zucker rats after bilateral cnXII transection. We also analyzed the data of the lean and obese animals as one group in protocol C. Figure 5 shows the effects of DOI on UA mechanics in eight cnXII-denervated rats compared with the results obtained in intact animals in protocol A (the data of lean and obese animals were combined). In cnXII-denervated animals (Figure 5), there were significant reductions in VImax (p = 0.007) and Pcrit (p = 0.017) and increases in Ron (p = 0.016) after DOI. The APcrit (-0.8 ± 0.3 cm H2O) in denervated animals was statistically smaller compared with the ΔPcrit (-2.8 ± 0.6 cm H2O) in animals with intact cnXII in protocol A (unpaired t test, p = 0.042). CnXII denervation did not affect the ΔVImax (-4.4 ± 1.2 ml/second in denervated vs. -1.8 ± 1.8 ml/second in intact animals, p = 0.377) or ARon (23.2 ± 7.4 cm H2O/L/second in denervated vs. 57.7 ± 12.8 cm H2O/L/ second in intact animals, p = 0.083). The ΔRon in denervated animals tended to be smaller, but the difference did not achieve statistical significance. The systolic BP significantly increased to 128 ± 12 mm Hg (p = 0.02), and diastolic BP increased to 83 ± 12 mm Hg (p = 0.014) after DOI. The administration of DOI did not affect heart rate. In all three protocols, systolic and diastolic BP increased to similar levels after DOI administration (Figures 4 and 5).
DISCUSSION
The major findings of this study are as follows. (1) Systemic administration of a 5-HT^sub 2A/2C^ receptor agonist rendered the UA less collapsible in both lean and obese Zucker rats, which was associated with an increased UA upstream resistance and unchanged VImax. (2) The improvement in UA stability induced by a 5-HT^sub 2A/2C^ receptor agonist administration is neuromuscular in origin and is predominantly, but not exclusively, mediated by hypoglossal motoneurons. (3) Nonhypoglossal motoneurons, at least in part, were responsible for mediating the increased Ron caused by the 5-HT^sub 2A/2C^ receptor agonist. (4) The magnitude of the changes in UA pressure-flow relationships induced by 5-HT^sub 2A/2C^ agonist is similar in lean and obese Zucker rats, although UA collapsibility in obese Zucker rats improved to levels comparable to the baseline values noted in lean animals. To our knowledge, this is the first study that investigated the effects of serotonergic stimulation on UA mechanics in the isolated UA preparation.
Critique of Method of Isolated UA
Before discussing our results, several limitations of the isolated UA preparation should be addressed. First, it is well known that anesthesia depresses respiration and reduces the GG muscle activity. In this study, the GG muscle exhibited faint activity at baseline (Figure 3), indicating that the level of anesthesia used did not totally suppress EMG^sub GG^. However, the level of anesthesia and end-tidal CO2 were maintained constantly throughout the experimental protocol. second, large negative suction pressures (-60 to -80 cm H2O) were generated to achieve airflow limitation in our preparation. It is conceivable that these pressures could traumatize the UA (15), leading to alterations in collapsibility. The UA is not actually exposed to such large negative pressure in vivo during normal physiologic activity. However, repeated measurements of UA collapsibility were shown to be reproducible with low coefficients of variation. Third, the vascular tone and mucosal blood volume have been suggested to be potentially important nonmuscular determinants of UA collapsibility. Wasicko and colleagues suggested that vasodilatation in cats increases UA collapsibility, whereas vasoconstriction tends to decrease airway collapsibility (16), although the degree of the effect on UA airflow mechanics, as well as the effects in other animal species, remains unclear. We found that DOI induced a significant increase in BP but not heart rate in all Zucker rats (Table 1), suggesting that DOI increased peripheral vascular lone. Therefore, in eight additional Zucker rats, we determined whether the DOI-induced increase in vascular tone affected UA mechanics. To isolate any potential secondary effect of the DOI-induced vasoconstriction on UA mechanics from the primary effects of DOI mediated through UA muscle activation, animals were paralyzed before assessing the impact of DOI on UA mechanics (protocol B). Despite similar increases in BP in these eight animals compared with the BP changes noted in our primary study, Pcrit was unaffected in paralyzed rats by DOI (Figure 4). This suggests that neither the vasoconstriction nor the increase of BP induced by DOI affected UA mechanics in our study.
Effects of DOI on UA Mechanics
Although ventilation may be normal during wakefulness in patients with obstructive sleep apnea (OSA), a sleep-induced reduction in UA dilator muscle activity results in collapse of an anatomically narrowed UA (17). It is thought that if UA dilator muscle activity can be maintained or augmented during sleep, then pharyngeal collapse may be prevented.
Serotonergic neurons exert an excitatory effect on UA dilator motoneurons (1, 18). Prior studies suggested that serotonergic agents may be effective in treating OSA (5, 6, 19-21). Indeed, administration of the serotonergic agents, trazodone and L-tryptophan, was effective in treating sleep-disordered breathing in the English bulldog, and the effectiveness of this therapy was related to increased UA dilator muscle activity during sleep (19). Of the 15 different 5-HT receptor subtypes that have been identified so far (22), the specific type of receptor that mediates the excitatory effects of 5-HT in UA motoneurons is thought to be of the 5-HT^sub 2A^ and 5-HT^sub 2C^ variety (1, 4, 5). We reasoned that it would be important to know the specific effects of the systemic administration of a 5-HT^sub 2A/C^ agonist on UA mechanics, especially because human studies involving selective serotonin reuptake inhibitors in the treatment of OSA have been disappointing (23, 24). We used a previously published isolated UA preparation (14). In this model of the UA as a Starling resistor system, Vimax is modulated by Pcrit and by the resistance upstream to the flow-limiting site. We predicted that VImax would increase with decreasing Pcrit after the administration of DOI because of increased activity of the GG muscle. In cats, Schwartz and coworkers showed that the bilateral electrical stimulation of the cnXII increased Vimax and decreased Pcrit, although this was offset by an increased upstream resistance, which was thought to be due to narrowing of the upstream segment (14). This increase in upstream resistance was minor, but likely attenuated the increase in VImax. In a preliminary study, we also found that bilateral supramaximal stimulation of the cnXII in Zucker rats increased VImax because of a more negalive Pcrit, although there also was a small increase in Ron (25).
In this study, the administration of DOI resulted in a very significant increase in EMG^sub GG^ activity that was associated with a decrease in UA collapsibility in bolh lean and obese Zucker rals, as expected. There was a concomitant large increase in Ron, associated with an unchanged VImax overall (Table 1). Our results suggest that the stimulation of motoneurons in the cnXII nucleus mediated the improvement in UA collapsibility observed with DOI, as we saw large increases in tonic activity of the GG muscle, an UA muscle solely innervated by cnXII (26-28). The relatively unaltered UA dynamics after DOI administration with complete neuromuscular paralysis (protocol B) supports the idea that the effect of DOI on UA collapsibility is neuromuscular in origin.
It is possible that DOI affected the reflex response to UA negative pressure, which may have altered UA collapsibility. The reflex response to UA negative pressure is an important aspect of cnXII motor control. The UA reflex increases cnXII neural output and genioglossal activity (29). However, Douse and White showed that 5-HT at the hypoglossal motor nucleus caused a large increase in tonic activity that had no effect on the cnXII reflex response to UA negative pressure in cats (30). They speculated that the large increase in tonic activity obscured the reflex increase in phasic cnXII activity, and the progressive saturation of cnXII motor output may have led to a diminished cnXII reflex response (30, 31). Therefore, it is unlikely that the reflex response to UA negative pressure played a major role to explain our results.
We also have to consider the effects of baroreceptor-mediated mechanisms on UA neuromuscular activity because DOI induced a significant increase in BP in all Zucker rats (Table 1). Increased BP increased the severity of UA airflow obstruction by increasing pharyngeal collapsibility (32, 33). Garpestad and colleagues found a decrease in EMG^sub GG^ activity during phenylephrine-induced hypertension (32). However, we found a large tonic activation of the GG muscle (Figure 3) with DOI. This large stimulation of cnXII nuclei activity may have offset any effects of the increased BP on UA collapsibility.
Serotonin is also known to affect UA muscle activity via peripheral mechanisms. It is possible that DOI affected the nodose ganglion because our animals were not vagotomized. Nodose ganglia, linking with the vagi, are the relay station between sensory neurons in the respiratory organs and central axons transmitting to the nucleus of the solitary tract of the medulla (34). However, 5-HT^sub 3^ receptors are likely the relevant receptors in the nodose ganglia, and 5-HT^sub 3^ antagonist rather than 5-HT^sub 3^ agonist increases cnXII activity (35, 36) through this mechanism. Therefore, we believe that the effects of DOI are not likely mediated through the nodose ganglia.
Role of cnXII in Mediating the Effects of DOI
Because DOI readily penetrates the blood-brain barrier and was administered systemically, we cannot exclude that other motoneuronal pools were also affected and could be partly responsible for our results in animals with intact cnXII. Therefore, to explore the relative role of cnXII in mediating the effects of DOI, we performed additional experiments involving cnXIIdenervated animals. Our results suggest that hypoglossal and nonhypoglossal tnotoneurons likely were involved in mediating the effects of DOI on UA mechanics. The fact that Pcrit decreased after DOI even in the denervated condition (protocol C) suggests that the effect of DOI on Pcrit seen in intact animals is not solely mediated through stimulation of the GG muscle but likely through other pharyngeal muscles as well. Other UA muscles are known to modulate UA patency (37-39), and we speculate that they were also activated by DOI. However, the decline in Pcrit in denervated animals was smaller than in intact animals and, therefore, the improvement in UA stability induced by DOI was attributed largely to activation of cnXII. These results are consistent with prior studies that suggest that the GG muscle is the main UA dilator muscle responsible for UA patency (40).
The large increases in Ron suggest that the UA upstream segment could have narrowed after DOI administration. There are several possible reasons to explain such narrowing of the upstream segment after DOI. First, increased activity of the EMG^sub GG^ could result in a lower mean intraluminal pressure in the upstream segment, resulting in a decreased cross-sectional area. With a more negative Pcrit after DOI, the pressure at the flow-limiting site can decrease, resulting in a larger pressure gradient across the upstream segment and, as a consequence, a lower mean intraluminal pressure, causing a narrowing of this segment (14). However, alterations in UA airflow dynamics with cnXII stimulation has been associated with minor increases in Ron with cnXII electrical stimulation in cats (14). It is unlikely, therefore, that this mechanism would be solely responsible for the large increases in Ron observed in our experiments. Second, the lateral branch of cnXII innervates the styloglossus and hyoglossus muscles (26, 27). These tongue retractors could have been simultaneously stimulated after systemic administration of DOI. However, because Ron also increased after transection of cnXII in protocol C, activation of tongue retractors after DOI likely is not the only explanation for the increased Ron in intact animals. Third, narrowing of the upstream segment could be due to increased recruitment of pharyngeal constrictors due to DOI. The pharyngeal constrictor muscles are sail-like muscles forming the lateral and posterior walls of the pharyngeal airway (41) and are innervated by the pharyngeal branch of the vagus and the glossopharyngeal nerve (42, 43). Kuna and Brennick showed that stimulation of the pharyngeal branch of the vagus decreased cross-sectional area and decreased compliance of the velopharynx in an isolated UA in decerebrate cats (27, 37). Indeed, the nucleus ambiguous, wherein the pharyngeal branch of the vagus originates, is known to contain 5-HT^sub 2A/2C^ receptors (44). Because DOI was administered systemically in our experiments, we speculate that motoneurons in the nucleus ambiguous were stimulated by DOI, resulting in decreased UA cross-sectional area and a decreased UA compliance. This may explain the decreased Pcrit and increased Ron in cnXH-denervated animals, as stimulation of the pharyngeal branch of the vagus by DOI could have affected UA cross-sectional area and stiffness.
Both electrical stimulation of cnXII and administration of serotonergic agents are being investigated as potential treatment strategies for OSA (23, 24, 45). In animals, electrical stimulation of cnXII has been reported to either have a minor (14) or no effect on Ron (46). This effect of cnXII electrical stimulation has been attributed to alterations in UA airflow dynamics rather than to a direct effect on UA muscles (14). Systemically administered 5-HT^sub 2A/2C^ agonist appears to exert a more complex effect on UA mechanics than isolated cnXII stimulation. Our results could have implications on the development of pharmacotherapy for OSA because both hypoglossal and nonhypoglossal motoneurons may possibly be modulated using serotonergic agents in this regard.
Obesity and UA Mechanics
In this study, the Pcrit of obese Zucker rats was significantly higher (less negative) compared with lean Zucker rats. This finding is consistent with our previous report (12) and indicates that the UA of obese Zucker rats is more collapsible compared with that of lean Zucker rats. Obesity did not modify the response to DOI because there were no significant differences in UA mechanics between lean and obese Zucker rats after DOI (Table 1). However, with increased recruitment of UA dilator muscle activity after DOI, the UA collapsibility of obese Zucker rats improved to levels comparable to the baseline values noted in lean animals. This suggests that augmentation of UA dilator muscle activity could indeed be useful in preventing collapse of the UA.
In conclusion, systemic administration of a 5-HT^sub 2A/2C^ receptor agonist appears to have complex effects on UA mechanics. Although DOI rendered the UA less collapsible, an increased UA upstream resistance and an unchanged VImax accompanied this effect. The decrease in Pcrit is likely mediated primarily through hypoglossal motoneurons, whereas the increase in upstream resistance may be due to a narrowing of the upstream segment that may be partly caused by pharyngeal constrictor stimulation. Obesity does not modify the UA response to the administration of a 5-HT^sub 2A/2C^ agonist, although UA collapsibility in obese Zucker rats improved to levels that are comparable to the baseline values of lean animals after significant EMG^sub GG^ activation by this drug. We believe that simultaneous examinations of UA muscle activity and UA mechanics in the isolated UA preparation offer an attractive way of determining the effects of other pharmacotherapeutic candidates for the treatment of OSA and help explain their potential mechanisms of action. Studies are also needed in unanesthetized animals to determine the effects of these agents on ventilation and recruitment of UA dilator muscles across sleep-wake states.
References
1. Kubin L, Tojima H, Davies RO, Pack AI. Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cal. Neurosci Lett 1992; 139:243-248.
2. Kubin L, Davies RO, Pack AI. Control of upper airway motoneurons during REM sleep. News Physiol Sci 1998;13:91-97.
3. Jelev A, Sood S, Liu H, Nolan P, Horner RL. Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep-wake states in rats. J Physiol 2001;532:467-481.
4. Zhan G, Shaheen F, Mackiewicz M, Fenik P, Veasey SC. Single cell laser dissection with molecular beacon polymerase chain reaction identifies 2A as the predominant serotonin receptor subtype in hypoglossal motoneurons. Neuroscience 2002;113:145-154.
5. Fenik P, Veasey SC. Pharmacological characterization of serotonergic receptor activity in the hypoglossal nucleus. Am J Respir Crit Care Med 2003; 167:563-569.
6. Veasey SC, Panckeri KA, Huffman EA, Pack AI, Hendricks JC. The effects of serotonin antagonists in an animal model of sleep-disordered breathing. Am .1 Respir Crit Care Med 1996;153:776-786.
7. Iida M, Murakami T, Ishicla K, Mizuno A, Kuwajima M, Shima K. Substitution at codon 269 (glutamine [arrow right] proline) of the leptin receptor (OB-R) cDNA is the only mutation found in the Zucker fatty (fa/fa) rat. Biochem Biophys Res Commun 1996;224:597-604.
8. Bray GA. The Zucker-fatty rat: a review. Fed Proc 1977;36:I48-153.
9. Farkas GA, Schlenkcr EH. Pulmonary ventilation and mechanics in morbidly obese Zucker rats. Am J Respir Crit Care Med 1994;150:356-362.
10. Magalang UJ, Farkas GA, Najdzionek JS, Nakano H. Obese Zucker rats have narrower upper airway compared to lean litter males [abstract]. Am J Respir Crit Care Med 2000; 161:A87.
11. Farkas GA, Gosselin LE, Zhan WZ, Schlenker EH, Sieck GC. Histochemical and mechanical properties of diaphragm muscle in morbidly obese Zucker rats. J Appl Physiol 1994;77:2250-2259.
12. Nakano H, Magalang UJ, Lee SD, Krasney JA, Farkas GA. Serotonergic modulation of ventilation and upper airway stability in obese Zucker rats. Am J Respir Crit Care Med 2001;163:1191-1197.
13. Ogasa T, Farkas GA, Michlin CP, Ray AD, Magalang UJ. Effects of serolonergic agonist on upper airway mechanics in obese Zucker rats [abstract]. Am J Respir Crit Care Med 2004;169:A431.
14. Schwartz AR, Thut DC, Russ B, Seelagy M, Yuan X, Brower RG, Permult S, Wise RA, Smith PL. Effect of electrical stimulation of the hypoglossal nerve on airflow mechanics in the isolated upper airway. Am Rev Respir Dis 1993;147:1144-1150.
15. Rowley JA, Williams BC, Smith PL, Schwarte AR. Neuromuscular activity and upper airway collapsibility. Mechanisms of action in the decerebrate cat. Am J Respir Crit Care Med 1997;156:5I5-521.
16. Wasicko MJ, Hull DA, Parisi RA, Neubauer JA, Mezrich R, Edelman NH. The role of vascular tone in the control of upper airway collapsibility. Am Rev Respir Dis 1990;141:1569-1577.
17. Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 1978;44:931-938.
18. Berger AJ, Bayliss DA, Viana F. Modulation of neonatal rat hypoglossal motoneuron excitability by serotonin. Neurosci Lett 1992;143:164-168.
19. Veasey SC, Fenik P, Panckeri K, Pack AI, Hendricks JC. The effects of trazodone with i.-tryptophan on sleep-disordered breathing in the English bulldog. Am J Respir Crit Care Med 1999;160:1659-1667.
20. Hudgel DW, Gordon EA, Meltzer HY. Abnormal scrotonergic Stimulation of Cortisol production in obstructive sleep apnea. Am J Respir Crit Care Med 1995;152:186-192.
21. Carley DW, Radulovacki M. Mirtazapine, a mixed-profile serotonin agonist/antagonisl, suppresses sleep apnea in the rat. Am J Respir Crit Care Med 1999;160:1824-1829.
22. Kroeze WK, Kristiansen K, Roth BL. Molecular biology of serotonin receptors structure and function at the molecular level. Curr Top Med Chem 2002;2:507-528.
23. Hanzel DA, Proia NG, Hudgel DW. Response of obstructive sleep apnea to fluoxetine and prolriptyline. Chest 1991;100:416-421.
24. Kraiczi H, Hedner J, Dahlof P, Ejnell H, Carlson J. Effect of serotonin uptake inhibition on breathing during sleep and daytime symptoms in obstructive sleep apnea. Sleep 1999;22:61-67.
25. Magalang UJ, Ray AD, Farkas GA. Effects of hypoglossal nerve stimulation on upper airway mechanics in obese Zucker rats [abstract]. Am J Respir Crit Care Med 2002;165:A798.
26. O'Reilly PM, FitzGcrald MJ. Fibre composition of the hypoglossal nerve in the rat. J Anat 1990;172:227-243.
27. Kuna ST, Brennick MJ. Effects of pharyngeal muscle activation on airway pressure-area relationships. Am J Respir Crit Care Med 2002;166:972977.
28. McClung JR, Goldberg SJ. Functional anatomy of the hypoglossal innervated muscles of the rat tongue: a model for elongation and protrusion of the mammalian tongue. Anat Rec 2000;260:378-386.
29. Mathew OP, Abu-Osba YK, Thach BT. Influence of upper airway pressure changes on genioglossus muscle respiratory activity. J Appl Physiol 1982;52:438-444.
30. Douse MA, White DP. Scrotonergic effects on hypoglossal neural activity and reflex responses. Brain Res 1996;726:213-222.
31. Eldridge FL, Gill-Kumar P, Millhorn DE. Input-output relationships of central neural circuits involved in respiration in cals. J Physiol 1981;311: 81-95.
32. Garpestad E, Basner RC, Ringler J, Lilly J, Schwartzstein R, Weinberger SE, Weiss JW. Phenylephrine-induced hypertension acutely decreases genioglossus EMG activity in awake humans. J Appl Physiol 1992;72: 110-115.
33. Mayor AH, Schwartz AR, Rowley JA, Willey SJ, Gillespie MB, Smith PL, Robotham JL. Effect of blood pressure changes on air flow dynamics in the upper airway of the decerebratc cat. Anesthesiology I996;84:128134.
34. Zhuo H, Ichikawa H, Heike CJ. Neurochemistry of the nodose ganglion. Prog Neurobiol 1997;52:79-107.
35. Fenik P, Ogawa H, Veasey SC. Hypoglossal nerve response to 5-HT3 drugs injected into the XII nucleus and vena cava in the rat. Sleep 2001; 24:871-878.
36. Carley DW, Radulovacki M. Role of peripheral serotonin in the regulation of central sleep apneas in rats. Chest 1999;115:1397-1401.
37. Kuna ST. Effects of pharyngeal muscle activation on airway size and configuration. Am J Respir Crit Care Med 2001;164:1236-1241.
38. Series F. Upper airway muscles awake and asleep. Sleep Med Rev 2002;6: 229-242.
39. Veasey SC. Molecular and physiologic basis of obstructive sleep apnea. Clin Chest Med 2003;24:179-193.
40. Oden M, Schnall R, Gavriely N, Oliven A. Dependency of upper airway patency on head position: the effect of muscle contraction. Respir Physiol 1995; 100:239-244.
41. Kuna ST, Smickley JS. Superior pharyngeal constrictor activation in obstructive sleep apnea. Am J Respir Crit Care Med 1997;156:874-880.
42. Bieger D, Hopkins DA. Viscerotropic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. J Camp Neural 1987;262:546-562.
43. Williams PL. Alimentary system: tongue and pharynx. In: Bannister LH, editor. Gray's anatomy. New York: Churchill Livingstone; 1995. p. 17211733.
44. Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin 1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neural 1995;351:357-373.
45. Schwartz AR, Bcnnetl ML, Smith PL, De Backer W, Hedner J, Boudewyns A, Van de Heyning P, Ejnell H, Hochban W, Knaack L, et al. Therapeutic electrical stimulation of the hypoglossal nerve in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg 2001;127:12161223.
46. Fuller DD, Williams JS, Janssen PL, Fregosi RF. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. J Physiol 1999;519:601-613.
Toshiyuki Ogasa, Andrew D. Ray, Charles P. Michlin, Caspar A. Farkas, Brydon J. B. Grant, and Ulysses J. Magalang
Department of Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine; Department of Exercise and Nutrition Sciences, University at Buffalo, Buffalo, New York; and Department of Medicine, Division of Pulmonary and Critical Care Medicine, The Ohio State University, Columbus, Ohio
(Received in original form December 9, 2003; accepted in final form July 8, 2004)
Supported by grants from Research for Health in Erie County, Inc., American Lung Association.
Correspondence and requests for reprints should be addressed to Ulysses ). Magalang, M.D., Division of Pulmonary and Critical Care Medicine, 201 Davis Heart and Lung Research Institute, 473 West 12th Avenue, Columbus, OH 43210. E-mail: magalang-1 @medctr.osu.edu
This article has an online supplement, which is accessible from this issue's table of contents online at www.atsjournals.org
Am J Respir Crit Care Med Vol 170. pp 804-810, 2004
Originally Published in Press as DOI: 10.1164/rccm.200312-1674OC on July 15, 2004
Internet address: www.atsjournals.org
Conflict of Interest Statement: T.O. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; A.D.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; C.P.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; C.A.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; B.J.B.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; U.J.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
Copyright American Thoracic Society Oct 1, 2004
Provided by ProQuest Information and Learning Company. All rights Reserved