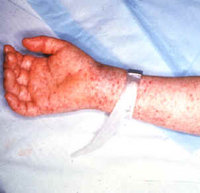
Rocky Mountain spotted fever (RMSF), a tickborne infection caused by Rickettsia rickettsii and characterized by a rash (Figure), has a case-fatality rate as high as 30% in certain untreated patients (1). Even with treatment, hospitalization rates of 72% and case-fatality rates of 4% have been reported (1-3). This report summarizes the clinical course of three fatal cases of RMSF in children and related illness in family members during the summer of 2003. These cases underscore the importance of 1) prompt diagnosis and appropriate anti-microbial therapy in patients with RMSF to prevent deaths and 2) consideration of RMSF as a diagnosis in family members and contacts who have febrile illness and share environmental exposures with the patient.
Case Reports
Oklahoma, In late May, a female child aged 7 years was taken to an emergency department (ED) with 2 days of fever (102.7[degrees] F [39.3[degrees] C]), malaise, abdominal pain, nausea, and vomiting. Viral gastroenteritis was diagnosed, and the patient was released. Four days later, the patient reported to a second ED with persistent fever, anorexia, irritability, photophobia, cough, diffuse myalgias, nausea, and vomiting. Physical examination showed hepatosplenomegaly and an erythematous papular rash with scattered petechiae on the trunk, arms, legs, palms, and soles. Laboratory results included an elevated white blood cell (WBC) count of 11.4 x [10.sup.9] cells/L (normal range: 3.0-9.1 x [10.sup.9] cells/L), thrombocytopenia (19 x [10.sup.9] platelets/L [normal range: 150-350 x [10.sup.9] platelets/L]), elevated aspartate aminotransferase (AST) of 279 U/L (normal: [less than or equal to] 42 U/L), and elevated alanine aminotransferase (ALT) of 77 U/L (normal: [less than or equal to] 48 U/L). In the ED, the patient was treated with intravenous (IV) doxycycline for suspected RMSF and transferred to a pediatric intensive care unit at a tertiary care medical center, where she had declining mental status, metabolic acidosis, and respiratory failure; the patient died 6 days after initially seeking treatment. IgG antibodies reactive with R. rickettsii at a reciprocal titer of 128 were demonstrated by using an indirect immunofluorescence antibody (IFA) assay in a serum specimen collected 2 days before death. Spotted fever group rickettsiae (SFGR) were detected by immuno-histochemical (IHC) staining at CDC in autopsy specimens from the brain, skin, heart, lung, spleen, and kidney.
On June 1, the child's sister, aged 3 years, had fever, headache, myalgias, and vomiting; on the following day, she had an erythematous maculopapular rash on the trunk, extremities, palms, and soles. RMSF was diagnosed, and the child was treated with doxycycline; she recovered. Seroconversion of IgG antibodies reactive with R. rickettsii was demonstrated in acute and convalescent phase serum specimens obtained during illness and 5 months later. Both children played frequently in grassy areas near their home. No history of tick bite was reported, although ticks were frequently observed on the family's per dogs and often were manually removed by members of the household.
Kentucky. In early August, a male child aged 2 years was taken to a pediatrician after 1 day of fever (101.0[degrees] F [38.3[degrees] C]) with a papular rash on his legs, arms, trunk, and back. An unspecified viral syndrome was diagnosed, and the child was treated with nonsteroidal anti-inflammatory drugs. During the next 2 days, the child continued to have fevers, spiking to 102.0[degrees] F-103.0[degrees] F (38.9[degrees] C-39.4[degrees] C), and variable rash. The child was examined in an ED and discharged with a diagnosis of viral infection. Four days after initial treatment, the child was again evaluated by a pediatrician because of lethargy and refusal to walk. Laboratory tests showed thrombocytopenia (42 x [10.sup.9] platelets/L), a WBC count of 3.3 x [10.sup.9] cells/L, anemia (hemoglobin 10.4 g/dL [normal range: 13.8-17.2 g/dL]), and hyponatremia (134 mmol sodium/L [normal range: 135-145 mmol sodium/L]). The next day, the child was admitted and treated with IV ceftriaxone and methyl-prednisolone. Two days later, the child was transferred to a tertiary care hospital. Physical examination at admission revealed a fine petechial rash on the groin, trunk, ankles, and palms. The patient was treated with IV vancomycin, cefotaxime, and doxycycline. His condition continued to deteriorate; 8 days after initial treatment, he died from multiple system failure. A serum specimen collected 2 days earlier tested positive by enzyme immunoassay for IgM antibodies reactive with R. rickettsii at 9.4 index value units (index values >2.0 were considered reactive by the testing laboratory). SFGR were detected by IHC stain in autopsy specimens of the brain, skin, heart, lung, spleen, kidney, lung, and adrenal gland.
The child's mother, aged 40 years, was hospitalized 2 days before her son's death with 2 days of diplopia, dizziness, headache, and fever. Oral doxycycline and IV ceftriaxone were administered; she was discharged after 5 days. Seroconversion of IgG antibodies reactive with R. rickettsii was demonstrated in acute and convalescent phase serum specimens obtained during illness and 2 weeks later. The family lived near a lake with woods. The mother did not recall any recent tick bites, travel, or participation in outdoor activities, by herself or her son prior to illness onset.
Arizona. In mid-August, a male child aged 14 months was taken to a community health clinic after 1 day of fever (103.7[degrees] F [39.8[degrees] C]), with a maculopapular rash, including the palms and soles, and thick white exudates on the tongue. Chest radiographic evaluation showed a possible right lower lobe infiltrate. The child was treated with intramuscular cefotaxime, acetominophen, and antifungal medication for presumptive thrush. The next day, the child visited the clinic with nausea, vomiting, anorexia, and dehydration. The patient was transferred to a referral hospital for treatment of pneumonia, roseola infantum, and thrush; on admission, the patient had a temperature of 105.7[degrees] F (41[degrees] C). After 3 days, he was transferred to a tertiary care hospital with a diagnosis of sepsis and disseminated intravascular coagulopathy. The patient was treated with IV ceftazidime and vancomycin. Laboratory findings included an elevated WBC count (16.2 x [10.sup.9] cells/L), thrombocytopenia (46 x [10.sup.9] platelets/L), and elevated levels of AST (291 U/L) and ALT (99 U/L). Six days after initial treatment, the child died of pulmonary hemorrhage; an autopsy was not performed. A serum specimen obtained 5 days before the child's death tested negative by IFA for IgM and IgG antibodies reactive with R. rickettsii; however, R. rickettsii DNA was amplified from serum by polymerase chain reaction (PCR) assay. A serum specimen obtained from a brother, aged 5 years, showed IgM and IgG antibodies reactive to R. rickettsii, indicating recent exposure. The children lived in a rural environment with low shrubs and grasses and frequently interacted with free-roaming dogs with ticks; however, neither child had a history of recent tick bite.
Editorial Note: RMSF is the most commonly fatal tickborne illness in the United States. Characterized by fever and a macular rash in its early stages, untreated RMSF can result in severe systemic manifestations, including pneumonitis, myocarditis, hepatitis, acute renal failure, encephalitis, gangrene, and death. An estimated 612 deaths were attributable to RMSF in the United States during 1983-1998, and approximately 12% of reported deaths occurred in children aged <10 years (4). Family clusters of infection area well-recognized feature of RMSF because of shared residence and risks for vector exposure (5).
In its early stages, RMSF can resemble many other infectious and noninfectious conditions and can be difficult to diagnose (Box), even for physicians familiar with the disease (3,6). The majority of patients do not have the classic RMSF triad of fever, rash, and history of tick bite on their first visit for medical care; often the rash appears several days after onset of fever and can evolve to become petechial. The absence of known tick bite is common and should not dissuade clinicians from suspecting RMSF. None of the patients in this report recalled a tick bite before illness onset, although all lived near wooded or grassy areas where ticks might have been present.
The infection can have a rapid course; 50% of RMSF deaths occur within 9 days of illness onset (1,2). Doxycycline therapy is considered the best treatment for RMSF in both adults and pediatric patients and is most successful when initiated within 5 days of illness onset (1,7). Delay of doxycycline therapy can increase the risk for severe or fatal outcomes; treatment should never be delayed pending laboratory confirmation.
Criteria for diagnosis * of a confirmed infection include the presence of a clinically compatible illness, plus at least one of the following: 1) serologic evidence of a significant change (fourfold increase of greater) in antibody titer reactive with R. richettsii antigens between paired serum specimens, as measured by a standardized assay conducted in a commercial, state, or reference laboratory; 2) demonstration of R. rickettsii antigen by IHC in a clinical specimen such as skin biopsy of other tissue; 3) detection of R. rickettsii DNA by PCR in a clinical specimen, such as whole blood or tissue; or 4) isolation of R. rickettsii from a clinical specimen in cell culture. Probable cases have a clinically compatible illness and serologic evidence of antibodies reactive with R. rickettsii in a single serum sample at a titer considered indicative of current of past infection (cutoff titers are determined by individual laboratories). At CDC, reciprocal IFA IgG titers of [greater than or equal to] 64 are considered to be evidence of current or past infection.
The most effective measures to reduce the risk for RMSF (particularly in children) are to 1) limit exposure to ticks during periods of peak tick activity (i.e., April-September); 2) inspect the head, body, and clothes for ticks thoroughly after being in wooded or grassy areas, especially along the edges of trails, roads, or yards; and 3) remove attached ticks immediately by grasping them with tweezers or forceps close to the skin and pulling gently with steady pressure. Because rapid laboratory confirmation of RMSF infection is not available, clinicians should consider initiating empiric therapy in patients with a compatible clinical presentation (e.g., fever usually with subsequent development of a macular or petechial rash) and epidemiologic circumstance (e.g., recent recreational or occupational activities during spring and summer months that could have exposed persons to ticks) to reduce morbidity and mortality resulting from delayed diagnosis (3,6). As a nationally notifiable disease, all RMSF cases should be reported to state health departments. Additional information about PMSF is available at http://www.cdc.gov/ncidod/dvrd/rmsf/index.htm.
BOX. Epidemiology, clinical findings, diagnosis, treatment, and prevention of Rocky Mountain spotted fever (RMSF)
Epidemiology
* RMSF is a zoonotic disease caused by the bacterium Rickettsia rickettsii and is transmitted to humans through the bite of the American dog tick (Dermacentor variabilis) and Rocky Mountain wood tick (D. andersoni).
* Cases have been reported from most states in the continental United States, most frequently from southeastern and south central states.
* Age-specific incidence is highest in children aged 1-9 years.
* Case-fatality rate is as high as 30% for certain untreated patients but decreases markedly with prompt and appropriate antibiotic treatment.
Clinical Findings
* Incubation period typically is 5-10 days after a tick bite.
* Early signs and symptoms are nonspecific and can include fever, nausea, vomiting, severe headache, muscle pain, and loss of appetite.
* Later signs and symptoms include abdominal pain, joint pain, and diarrhea.
* Rash is a frequent finding that usually occurs several days after onset of fever. Initial appearance of the rash usually is represented as faint macules on the wrists or ankles.
* As the disease progresses, the rash can become petechial and involve the trunk, extremities, palms, and soles.
* Laboratory abnormalities can include thrombocytopenia, hyponatremia, and elevations of hepatic aminotransferase levels.
* Severe manifestations can include pneumonitis, encephalitis, disseminated intravascular coagulopathy, and skin necrosis requiring amputation.
Diagnosis
* A working diagnosis primarily is based on clinical findings (e.g., fever and rash), seasonality (e.g., onset during April--September), and history of tick bite or tick exposure.
* Serologic tests for RMSF are available at commercial laboratories, stare public health laboratories, and CDC. Early serologic tests (within 1 week of illness onset) frequently are negative, and testing of acute and convalescent phase serum samples is recommended to confirm diagnosis.
* Nucleic acid detection (e.g., by using polymerase chain reaction assay), immunohistochemical staining of formalin-fixed tissues, and cell culture of biopsy or autopsy specimens also can be used for diagnosis and are available at specialized research laboratories and CDC.
Treatment
* Doxycycline is the treatment of choice for all patients.
--Dosage for adults is 100 mg twice daily.
--Dosage for children weighing <99 pounds (<45 kg) is 2.2 mg/kg twice daily; children weighing [greater than or equal to] 99 pounds should receive the adult dosage.
* Duration of therapy usually is 7-10 days; longer courses of therapy might be warranted in patients with more severe illness.
Prevention and Reporting
* RMSF is a nationally notifiable disease; cases should be reported to state health departments.
* No RMSF vaccine for humans is available.
* Prevention should focus on reducing exposure to ticks through avoidance of tick habitats and personal protective measures (e.g., tick checks and repellent).
* Additional information is available at http://www.cdc.gov/ncidod/dvrd/rmsf.
* A case definition for RMSF is available at http://www.cste.org/ps/2003pdfs/2003finalpdf/03-id-08revised.pdf.
References
(1.) Dalton MJ, Clarke MJ, Holman RC, et al. National surveillance for Rocky Mountain spotted fever, 1981-1992: epidemiologic summary and evaluation of risk factors for fatal outcome. Am J Trop Med Hyg 1995;52:405-13.
(2.) Treadwell T, Holman RC, Clarke MJ, Krebs JW, Paddock CD, Childs JE. Rocky Mountain spotted fever in the United States, 1993-1996. Am J Trop Med Hyg 2000;63:21-6.
(3.) O'Reilly M, Paddock C, Elchos B, Goddard J, Childs J, Currie M. Physician knowledge of the diagnosis and management of Rocky Mountain spotted fever: Mississippi, 2002. Ann NY Acad Sci 2003;990:295-301.
(4.) Paddock CD, Holman RC, Krebs JW, Childs JE. Assessing the magnitude of fatal Rocky Mountain spotted fever in the United States: comparison of two national data sources. Am J Trop Med Hyg 2002; 67:349-54.
(5.) Jones TF, Craig AS, Paddock CD, et al. Family cluster of Rocky Mountain spotted fever. Clin Infect Dis 1999;28:853-9.
(6.) Walker DH. Rocky Mountain spotted fever: a seasonal alert. Clin Infect Dis 1995;20:1111-7.
(7.) Kirkland KB. Wilkinson WE, Sexton DJ. Therapeutic delay and mortality in cases of Rocky Mountain spotted fever. Clin Infect Dis 1995;20:1118-21.
C Levy, MS, J Burnside, MS, T Tso, Arizona Dept of Health Svcs. S Englender, MD, M Auslander, DVM, S Billings, DVM, Div of Epidemiology and Health Planning, Kentucky Dept for Public Health. K Bradley, DVM, J Bos, MPH, L Burnsed, MPH, Div Communicable Diseases, Oklahoma Dept of Health. J Brown, MD, D Mahoney, MD, K Chamberlain, M Porter, C Duncan, B Johnson, R Ethelbah, K Robinson, M Wessel, S Savoia, MD, C Garcia, J Dickson, D Kvamme, D Yost, MD, M Traeger, MD, Indian Health Svc. J Krebs, MS, C Paddock, MD, W Shieh, MD, J Guarner, MD, S Zaki, MD, D Swerdlow, MD, J McQuiston, DVM, WL Nicholson, PhD, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; L Demma, PhD, EIS Officer, CDC.
COPYRIGHT 2004 U.S. Government Printing Office
COPYRIGHT 2004 Gale Group