These adhesives make strong, flexible bonds.
NASA's Jet Propulsion Laboratory, Pasadena, California
Adhesives that are blends of commercially available urethane and silicone adhesives have been found to be useful for bonding metal parts that flex somewhat during use. These urethane/silicone adhesives are formulated for the specific metal parts to be bonded. The bonds formed by these adhesives have peel and shear strengths greater than those of bonds formed by double-sided tapes and by other adhesives, including epoxies and neat silicones. In addition, unlike the bonds formed by epoxies, the bonds formed by these adhesives retain flexibility.
In the initial application for which the urethane/silicone adhesives were devised, there was a need to bond spring rings, which provide longitudinal rigidity for inflatable satellite booms, with the blades that provide the booms' axial strength. The problem was to make the bonds withstand the stresses, associated with differences in curvature between the bonded parts, that arose when the booms were deflated and the springs were compressed. In experiments using single adhesives (that is, not the urethane/silicone blends), the bonds were broken and, in each experiment, it was found that the adhesive bonded well with either the ring or with the blade, but not both. After numerous experiments, the adhesive that bonded best with the rings and the adhesive that bonded best with the blades were identified. These adhesives were then blended and, as expected, the blend bonded well with both the rings and the blades.
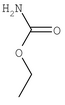
The two adhesives are Kalex (or equivalent) high-shear-strength urethane and Dow Corning 732 (or equivalent) silicone. The nominal mixture ratio is 5 volume parts of the urethane per 1 volume part of the silicone. Increasing the proportion of silicone makes the bond weaker but more flexible, and decreasing the proportion of silicone makes the bond stronger but more brittle.
The urethane/silicone blend must be prepared and used quickly because of the limited working time of the urethane: The precursor of the urethane adhesive is supplied in a two-part form, comprising a resin and a hardener that must be mixed. The resulting urethane adhesive has a working time of 3 to 5 minutes. To prepare the urethane/silicone blend, one must quickly add the silicone to the urethane adhesive and mix it in thoroughly within the working time of the urethane.
Once the urethane/silicone blend has been mixed and applied to the bond surfaces, it takes about 2 hours for the adhesive to cure under pressure. However, it takes about 24 hours for the adhesive to reach full strength.
This work was done by Paul D. Edwards of Callech for NASA's Jet Propulsion Laboratory. For further information, access the Technical Support Package (TSP) free on-line at www.techbriefs.com/tsp under the Materials category.
In accordance with Public Law 96-517, the contractor has elected to retain title to this invention. Inquiries concerning rights for its commercial use should be addressed to:
Innovative Technology Assets Management
JPL
Mail Stop 202-233
4800 Oak Grove Drive
Pasadena, CA 91109-8099
(818) 354-2240
E-mail: iaoffice@jpl.nasa.gov
Refer to NPO-30737, volume and number of this NASA Tech Briefs issue, and the page number.
Get information on the latest Composites/Plastics products by visiting our Technology Focus Products page online at www.techbriefs.com/techfocus. From there, link directly to each vendor's Web site for more information.
Copyright Associated Business Publications May 2004
Provided by ProQuest Information and Learning Company. All rights Reserved