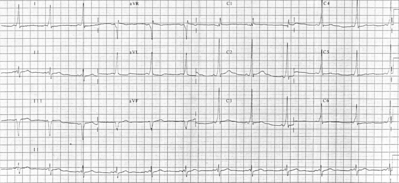
Electrophysiologic study was performed in a 52-year-old man with type A ventricular preexcitation. An accessory atrioventricular pathway with no ventriculoatrial conduction was localized to the posteroseptal region. "Fatigue phenomenon," defined as suppression of atrioventricular conduction following rapid pacing, was observed to be provoked by atrial pacing in a rate- and duration-dependent manner. Administration of 5 mg of intravenous verapamil during sinus rhythm abolished the delta waves. These observations may indicate that pathologic changes in the accessory pathway are responsible for these phenomena.
Depression of atrioventricular conduction following rapid pacing has been defined as the "fatigue phenomenon."[1] This phenomenon has been demonstrated in the abnormal conduction system.[1-3] Verapamil, a calcium-channel blocker, has essentially no electrophysiologic effect on an accessory pathway made up of sodium-channel-dependent myocardium.[4] Suppression of conduction with a calcium-channel-blocking drug would indicate the presence of a slow inward calcium-sensitive current. In this report, we describe a case in which verapamil abolished anterograde conduction of an accessory pathway that exhibited the fatigue phenomenon.
CASE REPORT
A 52-year-old man was admitted for evaluation of syncope. He had long-standing ECG evidence of type A ventricular preexcitation. There was no documentation of intermittent preexcitation. He had no organic heart disease as assessed clinically and echocardiographically. Electrophysiologic study was performed with the patient in the mildly sedated, postabsorptive state after informed consent had been obtained. The patient was receiving no antiarrhythmic drugs at the time of the study. The techniques for electrophysiologic study have been described previously.[5]
Quadripolar electrode catheters were positioned in the high right atrium, coronary sinus, and right ventricular apex and across the tricuspid valve to record the His bundle potential. Intracardiac electrograms and multiple surface ECG leads were displayed simultaneously. The heart was stimulated at twice the diastolic threshold current with a pulse width of 2 ms. Sinus node recovery times and the refractory periods of the right atrium, the atrioventricular node, and the right ventricle were normal. There was no ventriculoatrial conduction at any cycle length tested. An accessory atrioventricular pathway capable of anterograde conduction only was localized to the posteroseptal region. At pacing cycle lengths of 600 and 500 ms, the anterograde effective refractory periods of the accessory pathway were 360 and 390 ms, respectively. During incremental atrial pacing, 1:1 conduction over the accessory pathway persisted to 460 ms, with conduction block in the pathway at 450 ms. The interval between the pacing stimulus and a delta wave was always constant without any evidence of "decremental properties" in the accessory pathway.[6] Intermittent preexcitation never developed (sinus cycle lengths = 750 to 800 ms) in the control state. Fatigue phenomenon was observed following rapid atrial pacing (Fig 1). During and after atrial pacing at a cycle length of 660 ms for 30 s, delta waves persisted. However, three consecutive normal QRS complexes were noted upon termination of atrial pacing at a cycle length of 400 ms for 30 s. Furthermore, atrial pacing at a cycle length of 320 ms for 30 s was followed by 12 consecutive normal QRS complexes. The loss of delta waves was also related to the duration of the atrial pacing. Pacing at comparable cycle lengths for 5 s or less was followed by no more than two normal QRS complexes. However, it was not related to the sinus cycle length. Ventricular pacing failed to provoke this phenomenon. Subsequently, 5 mg of verapamil was administered intravenously during sinus rhythm. Approximately 2 min later, the delta waves were abolished (Fig 2). Repeat atrial stimulation showed no evidence of anterograde accessory pathway conduction at any rate.
DISCUSSION
In a previous study, Narula and Runge[1] showed that the fatigue phenomenon, defined as suppression of atrioventricular conduction following rapid pacing, was observed in the diseased His-Purkinje system. Fisch[2] demonstrated three cases of bundle branch block due to ventricular tachycardia and speculated that the phenomenon may be related to "fatigue" or "overdrive suppression." All of his patients had advanced heart disease. Ohe and his colleagues[3] reported a similar phenomenon in an accessory pathway elicited by rapid ventricular pacing in a 43-year-old man with the Wolff-Parkinson-White syndrome. As in our case, the anterograde electrophysiologic properties of the accessory pathway were moderately prolonged. To the best of our knowledge, their case is the only one in which this phenomenon occurred in an accessory pathway. Our case extends their observation and demonstrates that this phenomenon can also be provoked into an accessory pathway by atrial pacing in a rate-and duration-dependent manner. Since all QRS complexes normalized during rapid atrial pacing (Fig 1), one can postulate that this phenomenon was also the result of repetitive retrograde concealed conduction into the accessory pathway via the atrioventricular node. There was no evidence of ventriculoatrial conduction in our case. Retrograde concealed conduction has been demonstrated to occur even in the accessory pathway capable of anterograde conduction only.[7] However, failure to provoke the fatigue phenomenon with ventricular pacing in our case suggests that anterograde input into the accessory pathway might be largely responsible for this phenomenon for at least sinus cycle lengths tested. Additional pacing modalities, such as atrial pacing after ventricular pacing, might have been of help in further elucidating the contribution of retrograde concealment for other settings. Additionally, stable sinus cycle lengths after pacing may render bradycardia-dependent block in the accessory pathway less likely as the mechanism. Whatever the mechanisms involved, it appears that this phenomenon is associated with the diseased atrioventricular conduction system.[1-3]
Our case demonstrated another interesting phenomenon--total abolition of accessory pathway conduction after intravenous administration of verapamil. Generally, the accessory pathways are made up of muscle strands that are sodium-channel-dependent.[4] Thus, this agent has minimal or no effect on the electrophysiologic properties of the accessory pathway.[4] Conversely, conduction block in an accessory pathway after verapamil would suggest that the pathway is made up of cells that are different from normal myocardium. In 2 of their 39 patients with accessory pathways, Tai et al[8] showed that verapamil markedly prolonged the ventricular conduction time and the refractory periods of accessory pathways that had "decremental conduction properties" and were capable of retrograde conduction only. They indicated that these pathways may contain cells similar to those of the atrioventricular node. Horio and his associates[9] reported catecholamine-sensitive accessory pathways in two patients with intermittent ventricular preexcitation provoked by exercise. Verapamil completely abolished preexcitation in both cases. Our case also illustrates that a calcium-channel blocker can block accessory pathway conduction.
Cellular eletrophysiologic studies using ventricular myocardium resected from patients with ischemic heart disease[10] demonstrated that verapamil could suppress some action potentials in damaged myocardium. This finding suggests that such myocardium is dependent on a slow inward calcium-sensitive current. Although totally speculative, such pathologic changes, including degenerative changes in the accessory pathway,[11] may be responsible for altered electrophysiologic phenomena, such as both the fatigue phenomenon and conduction block with verapamil.
REFERENCES
[1] Narula OS, Runge M. Accommodation of A-V nodal conduction and fatigue phenomenon in the His-Purkinje system. In: Wellens HJJ, Lie KI, Janse MJ, eds. The conduction system of the heart. Leiden, The Netherlands: H. E. Stenfert Kroese, 1976; 529-44
[2] Fisch C. Bundle branch block after ventricular tachycardia: a manifestation of "fatigue" or "overdrive suppression." J Am Coll Cardiol 1984; 3:1562-64
[3] Ohe T, Shimomura K, Shiroeda O. Fatigue phenomenon of the accessory pathway. Int J Cardiol 1985; 8:211-14
[4] Harper RW, Whitford E, Middlebrook K, Federman J, Anderson S, Pitt A. Effects of verapamil on the electrophysiologic properties of the accessory pathway in patients with the Wolff-Parkinson-White syndrome. Am J Cardiol 1982; 50:1323-29
[5] Fujimura O, Kuo CS, Smith BA. Pre-excited RR intervals during atrial fibrillation in the Wolff-Parkinson-White syndrome: influence of the atrioventricular node refractory period. J Am Coll Cardiol 1991; 18:1722-26
[6] Klein GJ, Prystowsky EN, Pritchett ELC, David D, Gallagher JJ. Atypical patterns of retrograde conduction over accessory atrioventricular pathways in the Wolff-Parkinson-White syndrome. Circulation 1979; 60:1477-86
[7] Kawara T, Suzuki F, Ohtomo K, Sato T, Yamamoto N, Tanaka K, et al. Demonstration of retrograde concealed conduction in an accessory atrioventricular pathway capable only of anterograde conduction [in Japanese]. Clin Electrophysiol 1990; 13:99-109
[8] Tai DY, Chang MS, Svinarich JT, Chiang BN, Sung RJ. Mechanisms of verapamil-induced conduction block in anomalous atrioventricular bypass tracts. J Am Coll Cardiol 1985; 5:311-17
[9] Horio Y, Matsuyama K, Morikami Y, Rokutanda M, Hirata A, Okumura K, et al. Blocking effect of verapamil on conduction over a catecholamine-sensitive bypass tract in exercise-induced Wolff-Parkinson-White syndrome. J Am Coll Cardiol 1984; 4: 186-91
[10] Gilmour RF, Heger JJ, Prystowsky EN, Zipes DP. Cellular electrophysiologic abnormalities of diseased human ventricular myocardium. Am J Cardiol 1983; 51:137-44
[11] Klein GJ, Hackel DB, Gallagher JJ. Anatomic substrate of impaired conduction over an accessory pathway in the Wolff-Parkinson-White syndrome. Circulation 1980; 61:1249-56
COPYRIGHT 1993 American College of Chest Physicians
COPYRIGHT 2004 Gale Group