ABBREVIATIONS: BCE = before the common era; BSL = biosafety levels; CDC = Centers for Disease Control and Prevention; CE = common era; EEISA = enzyme-linked immuiiosorbent assay; EMB = eosin methylene blue agar; MAC = MacConkey agar; WHO = World Health Organization; Yops = Yersinia outer proteins.
INDEX TERMS: bioterrorism; black death; buboes; plague; Yersinia pestis.
Clin Tab Sci 2004;17(1):25
Yersinia. pestts, the causative agent of plague, is an aerobic, non-motile, gram-negative bacillus belonging to the family Enterobacteriacea. It is a zoonotic infection transmitted to humans via the bite of a flea. Three clinical forms of human plague exist: bubonic, pneumonic, and septicemic. Many important virulence factors associated with this organism are responsible for its extreme pathogenicity and high mortality rates. The bubonic form of plague is usually not transmitted human to human but the pneumonic form is - through inhalation of contaminated aerosol droplets. The pneumonic plague would be the form most likely implicated in the event of an intentional attack. Inhalation of aerosols can cause devastating consequences resulting in many casualties. Unless antibiotics are administered within 24 hours of the initial symptoms, death is inevitable. Its potential for use as a biological weapon is of major concern to public health officials.
LEARNING OBJECTIVES
See learning objectives #1 through #7 on page 40.
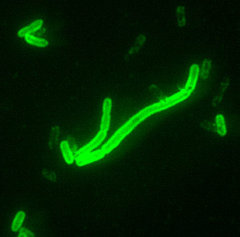
Yersinia pestis, the causative agent of plague, is an aerobic, non-motile, gram-negative bacillus belonging to the family Enterobacteriacea. It is approximately 0.5 to 0.8 µm in width by 1 to 3 µm in length 1,2 and demonstrates bipolar staining (closed safety pin appearance) with Giemsa, Wright's, or Wayson stains.1,3 The characteristic bipolar staining is usually not visible when stained with Gram Stain.1 Colonies are greywhite, pinpoint, and non-hemolytic on sheep blood agar after 24 hours of incubation. It is catalase positive, and oxidase and urease negative. Although it grows at 35 °C to 37 °C, its optimal growth is room temperature, approximately 28 °C.1-3
The first outbreak of plague was documented in 1320 before the common era (BCE) among the Philistines. Biblical reference to this outbreak is found in I Samuel 5:6. In the last two millennia, the plague has reached pandemic proportions affecting many countries on most continents.4
The first plague pandemic, also referred to as the Justinian Plague, began in Pelusium, Egypt in 541 common era (CE).4-6 Outbreaks in Europe, Central and Southern Asia, and Africa killed an estimated 100 million people.4,5
The second pandemic of the fourteenth century (1347 - 1350 CE) began in Sicily and rapidly spread throughout Europe over the next several years, killing an estimated one third of the European population.4-6 During that time, plague became known as "Black Death". Outbreaks of plague continued to occur sporadically in Europe over the next several centuries. The third pandemic began in 1894 in Hong Kong and Canton and in Bombay in 1898. It spread around the world over a ten year period, predominantly from infected rats and their fleas that were aboard steamships.4,5 An estimated 12 million deaths occurred. Between 1898 and 1908 approximately six million deaths occurred in India alone.7 Although large outbreaks are now rare, cases of plague are still reported in endemic areas such as the southwestern U.S. In the event a case occurs in the eastern U.S., it should be reported immediately to the proper authorities.
The natural reservoir of Y.pestis is rodents, squirrels, and prairie dogs. It is usually transmitted via the flea Xenopsylla cheopis.8 Two epidemic forms of the disease occur: urban plague and sylvatic plague. The urban plague involves the urban rat population and has been implicated in a number of human outbreaks.2 The last reported outbreak of urban plague occurred in Los Angeles between 1924 and 1925.9 The wild rodent population, such as mice, rabbits, rats, and prairie dogs, carries the sylvatic plague, which is endemic in the western U.S.2,10 The WHO reports 1,000 to 3,000 cases of plague worldwide each year.
CLINICAL INFECTIONS AND MANIFESTATIONS
Three clinical forms of human plague exist depending on the mode of entry: bubonic (lymph nodes), pneumonic (respiratory), and septicemic (blood). The pathogenicity and virulence of this organism has been attributed to numerous virulence factors such as: F1 antigen, plasminogen activator, a hemin storage system, V and W antigen's, Yersinia outer proteins (Yops proteins), lipopolysaccharide endotoxin, and phospholipase D.5,11,12 These factors enable the organism to survive within the host by utilizing essential nutrients and avoiding phagocytosis as well as other immune mechanisms triggered during the immune response.5,11,13,14
Bubonic plague
Of the three clinical forms of plague, the bubonic form is the most common. The infection occurs as the result of a bite from an infected flea or handling infected animals. Sudden onset of fever, headache, chills, nausea, vomiting, abdominal pain, and weakness occur between two to eight days after inoculation of the organism.15-17 The organism travels via the lymphatic system to the nearest lymph nodes. One to two days later, a painful lymphadenitis results causing the infected lymph nodes to become tender and swollen (called buboes). Other organs affected may include the skin, liver, and spleen.17 The buboes usually form in lymph nodes located in the cervical, axillary, and groin areas and are one to ten cm in diameter. The skin overlying the bubo is tender, red, warm, and often swollen with extensive edema. These regions become so inflamed and painful, the patient may no longer be able to physically move the infected area.5,16
The bubonic form of the plague is not transmitted from person to person. Approximately 5% to 15% of patients suffering from bubonic plague will develop secondary pneumonic plague. This occurs if the bacterium travels through the blood stream to the lungs. Once in the lungs, the organism can be transmitted person to person via inhalation of respiratory droplets.8,16
Pneumonic plague
Two forms of pneumonic plague exist. Primary pneumonic plague and secondary pneumonic plague. Primary pneumonic plague occurs as a result of inhalation of contaminated aerosols and is transmitted person to person. Symptoms are often acute in onset some two to three days after inhalation of the plague bacilli. These include fever, headache, weakness, cough, a bloody or watery sputum, chest pain, and dyspnea.5,8,16,17 These symptoms rapidly progress to pneumonia and may ultimately lead to respiratory failure if antibiotics are not administered within 24 hours.8
Secondary pneumonic plague develops in a small percentage of individuals diagnosed with bubonic or septicemic plague. The organism gains access to the blood stream and enters the lungs. Once in the lungs, symptoms include severe bronchopneumonia, shortness of breath, cough, chest pain, and bloody sputum in addition to those attributed to buboes.8,13
The pneumonic form of the plague is characterized by a shorter incubation period (two to three days compared to two to eight days in the bubonic form) and has a greater mortality rate. Both forms of pneumonic plague are transmitted from person to person and therefore are highly communicable.
Septicemic plague
Septicemic plague may occur primarily or as a complication of pneumonic or bubonic plague. When septicemic plague occurs by itself, it is caused in the same way as the bubonic plague except with the absence of buboes.15 After a one to four day incubation period, symptoms may include fever, shock, disseminated intravascular coagulation, cyanosis and necrosis, purpura, and gangrene.8,15,16 Gangrene of the fingers, toes, and nose is common hence the term "black death".5 Septicemic plague is not transmitted person to person.
PATHOGENESIS
The pathogenicity and high mortality rates of Y. pestis have been attributed to the presence of virulence factors. These factors allow the organism to propagate and acclimate to the host's internal environment. The F1 antigen has antiphagocytic properties and is the component responsible for eliciting a humoral immune response. This antigen therefore serves as a target for serological based diagnostic tests. Plasminogen activator is a protease responsible for the degradation of fibrin (fibrinolysin) and other extracellular proteins, which contribute to the systemic spread of the organism from the initial inoculation site. A hemin storage system enhances survival in phagocytic cells, increases uptake into eukaryotic cells, and has been selected as a marker of the organism's pigmentation system in the laboratory. The V and W antigens enable the organism to resist phagocytosis; the V antigen is also essential for the existence of Y pestis in macrophages.5,11,12
Interestingly, it has been established that both the F1 antigen and the V and W antigens are necessary for virulence but are only produced when the organism is grown at 37 °C, not at lower temperatures. This might explain why the flea, whose normal body temperature is approximately 25 °C is unaffected by this organism.18 Yops proteins are responsible for inhibiting platelet aggregation, phagocytosis, and blocking an effective inflammatory response. Lipopolysaccharide endotoxin (encoded on the chromosome) causes the classic features of endotoxic shock. Phospholipase D (PLD) allows the bacilli to survive in the flea midgut.5,11,12
CATEGORYAND BIOSAFETY LEVELS
Yersiniapestis is classified by the CDC as a Category 'A' organism. Due to the fact the organism is extremely virulent as well as pathogenic, BSL-2 practices, equipment, clothing, and facilities are recommended for the handling and processing of clinical specimens. Since manipulation of positive cultures may result in aerosol release, BSL-3 practices are required.3,19
SPECIMEN COLLECTION
Appropriate specimens for analysis include blood cultures, bubo aspirates, sputum, cerebral spinal fluid, and scrapings from skin lesions.5 Blood cultures should be collected according to the customary laboratory protocol and transported at room temperature. Sputum should be collected in a sterile container. If a delay in transport is unavoidable and specimen transport time is greater than one hour, samples should be sent at refrigerated temperatures. Bubo aspirates are obtained using a 20-gauge needle and a 10 mL syringe containing one to two mL of sterile saline for infusing the node and should be transported to the laboratory immediately.14
DIAGNOSIS
Diagnosis is based on the patient's history including environmental exposure history. A presumptive diagnosis can be made quickly based on symptoms and concomitant laboratory results. Blood, bubo aspirates, and sputum should be obtained from patients in suspected cases. Table 1 represents the CDC's guidelines for diagnosis of Y. pestis in Level A laboratories.20 Approved testing in Level B laboratories include direct immunofluorescence (DFA) based on the F1 antigen and serological diagnosis using either passive hemagglutination or an Fl antigen capture ELISA.21 The F1 antigen capture ELISA method has been described with 100% sensitivity in bubo specimens, 52% in serum specimens, and 58% in urine specimens.22 Paired sera demonstrating a four fold rise in titer between acute and convalescent samples is considered confirmatory. In addition, positive results using the polymerase chain reaction are also confirmatory.23
Researchers with the Pasteur Institute in Paris, France have developed a rapid diagnostic test for the plague bacteria. The methodology utilizes monoclonal antibodies to the F1 antigen in a simple dipstick assay yielding results with 100% sensitivity and specificity.24 "This new rapid test produced reliable results within 15 minutes at remote clinical facilities," noted Dennis and Chu of the CDC.25 In the event of an actual attack, this diagnostic test can provide the rapid identification needed for prompt administration of appropriate treatment.
BIOLOGICAL WEAPON
As far back as the mid 1300s, plague has been used as a biological weapon. During World War II, the apparent use of plague as a bioweapon by the Japanese in China produced certain criteria for medical officers to consider in differentiating endemic plague from an intentional release. It was reported that Japanese planes dropped rice and wheat grains mixed with fleas over cities in China. Weeks later, according to the report, plague appeared for the first time in cities where the disease was not reported before. There was also no evidence of epizootic or increased mortality among the rat population.26 Its use in the 21st century is a harsh reality that healthcare professionals must be prepared to handle and contain in an intentional attack. The bubonic form of the plague is naturally occurring and is endemic in the southwestern part of the U.S. The pneumonic form, due to its aerosol spread, would most likely be the form implicated in a terrorist attack. Its release into the environment would go undetected since it is odorless, colorless, and tasteless.27 Unfortunately, due to the gap between exposure and manifestation of symptoms, individuals infected not only run the risk of exposing people in close proximity but also, if travelers, can introduce the deadly organism to different parts of the country or the world causing an epidemic/pandemic if not contained immediately. Due to its extreme virulence and pathogenicity many casualties may result unless antibiotics are administered within 24 hours of the first symptoms. If pneumonic plague is suspected, local and state health departments must be notified immediately. If bioterrorism cannot be ruled out, they in turn should notify the CDC, the FBI, and any other appropriate authorities. An immediate response and proper containment may prevent an outbreak and ultimately save thousands of lives. Measures taken to contain a biological attack would be quarantine of infected individuals, prophylactic therapy, and restricted travel.27
VACCINES AND TREATMENT
Early treatment of pneumonic plague is essential for patient survival. As stated, antibiotics must be administered within 24 hours of the first symptoms. Streptomycin, gentamicin, the tetracyclines, and chloramphenicol are all effective antibiotics against the plague.15 Treatment should be administered for a minimum often days.8
Patients suffering from the bubonic form of the plague should be placed in isolation for a minimum of two days after administration of antimicrobial therapy to avoid the potential spread of the disease.5 Patients with the pneumonic form of the disease should be isolated until they have had at least four days of antimicrobial therapy.8
A dead or inactivated vaccine was available in the U.S. until 1998, when it was discontinued by its manufacturers.10 Currently, no vaccine is available for the plague. Research is in progress but a vaccine is not expected to be accessible for several years.
PREVENTION
If a person comes in close contact with an infected individual, chances of developing symptoms are greatly reduced if prophylactic therapy is instituted within seven days of exposure.8,10 Individuals exposed to either the bubonic form or pneumonic form should be quarantined as a precautionary measure. Control of the urban rat population and elimination of their fleas may also prevent the spread of human disease.
CONCLUSION
Yersinia pestis, the causative agent of plague, can be a fatal disease if treatment is not administered promptly. Due to its extreme virulence, its use as a biological weapon is of utmost importance to public health officials. Since the pneumonic form of the plague is odorless, colorless, and tasteless many casualties are possible if containment measures are not put into effect immediately.
REFERENCES
1. Sneath PH, Mair NS, Sharpe ME, Holt JG, editors. Bergeys manual of systematic bacteriology. Vol 2. Baltimore Md: Williams & Wilkins; 1986.
2. Koneman EW, Allen SD, Janda WM, and others. Color atlas and textbook of diagnostic microbiology. 5th ed. Philadelphia PA: Lippincott-Raven; 1997. p 215.
3. Carroll K, Held M, Stombler RE, and others. Laboratory preparedness for bioterrorism: from the phlebotomist to the pathologist. Lab Med 2003;3(34):169-80.
4. Plague. In: WHO report on global surveillance of epidemic-prone infectious diseases, 2000 http://www.who.int/emc-documents/surveillance/docs/whocdscsrisr2001.html/plague/plague.htm#History. Accessed May 28 2003.
5. Perry RD, Fetherston JD. Yersinia pestis-etiologic agent of plague. Clin Microbiol Rev 1997;10:35-66.
6. Center for Civilian Biodefense Strategies: Plague fact sheet info http:/ /www.hopkins-biodefense.org/pages/agents/agentplague.html. Accessed May 12 2003.
7. Datta KK. Plague epidemiology, prevention and control. National Institute of Communicable Diseases, Delhi India, 1994.
8. Shih C-L, Shih F-Y. Plague in bioterrorism. Ann Disaster Med: VoI 1 Suppl 1 2002.
9. CDC Plague Home page, http://www.cdc.gov/ncidod/dvbid/plague/ index.htm. Accessed May 12 2003.
10. Center for the Study of Bioterrorism: St Louis University School of Public Health: http://www.bioterrorism.slu.edu/. Accessed May 12 2003.
11. Dennis D, Meier F. Plague. In: Horsburgh CR, Nelson AM, editors. Pathology of emerging infections. Washington DC: ASM Press; 1997. p 21-47.
12. McGovern TW, Friedlander AM. Plague. In: Zajtchuk R, Bellamy RF, editors. Textbook of military medicine: medical aspects of chemical and biological warfare. Washington DC: Office of the Surgeon General, Borden Institute, Walter Reed Army Medical Center; 1997.
13. Butler T. Yersinia species (including plague). In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. New York NY: Churchill Livingstone; 1995. p 2070-8.
14. McGovern TW, Friedlander A. Plague. In: Zajtchuk R, Bellamy RF, editors. Medical aspects of chemical and biological warfare. Bethesda Md: Office of the Surgeon General; 1997. p 479-502.
15. CDC Public Health Emergency Preparedness & Response: facts about pneumonic plague: http://www.bt.cdc.gov/documentsapp/ FactShcet/Plague?about.asp. Accessed May 12 2003.
16. Inglesby TV, Dennis DT, Henderson DA, and others. Working Group on Civilian Biodefense. Plague as a biological weapon: medical and public health management. JAMA 2000;283(17):2281-90.
17. Deployment Health Clinical Center War on Terrorism - Biologic Warfare - Plague, http://www.pdhealth.mil/wot/plague.asp. Aceessed May 12 2003.
18. Center for Environmental Health and Safety - Southern Illinois University Carbondale. http://www.cehs.siu.edu/fix/medmicro/ yersi.htm. Accessed May 12 2003.
19. Carroll K, held M. Laboratory preparedness for bioterrorism. ASCP Teleconference. March 4, 2003.
20. Laboratory response network level a laboratory procedures for identification of Y. pestis. http://www.bt.cdc.gov/Agent/Plague/ ype_la_cp_121301.pdf. Accessed May 12 2003.
21. Forbes BA, Sahm DF, Weissfeld AS. Bailey & Scott's diagnostic microbiology. 11th edition. St Louis MO: Mosby Inc 2002. p 373.
22. Chanteau S, Rahalison L, Ratsitorahina M, and others. Early diagnosis of bubonic plague using F1 antigen capture ELISA assay and rapid immunogold dipstick. Int J Med Microbiol 2000;290(3):279-83.
23. Norkina OV, Kulichenko AN, Gintsburg AL, and others. Development of a diagnostic test for Yersinia pestis by the polymerase chain reaction. J Appl Bacteriol 1994;76(3):240-5.
24. Stephenson J. Plague rapid diagnostic test. JAMA 2003;289(6):691.
25. Dennis D, Chu M. A major new test for plague. Lancet 2003;353:361:191-2.
26. Zajtchuk R, editor. Textbook of military medicine: medical aspects of chemical & biological warfare. Washington DC, Office of the Surgeon General, Dept of the Army, USA. Walter Reed Army Medical Center 1998. p 485.
27. Biohazard News Disease Agents: Plague. http:// www.biohazardnews.net/agent_plague.shtml#bioterror. Accessed May 12 2003.
Deborah Josko MS CLS(M) is Assistant Professor at the University of Medicine and Dentistry of New Jersey, Newark NJ.
Address for correspondence: Deborah Josko MS CLS(M), Assistant Professor, University of Medicine and Dentistry of New jersey, School of Health Related Professions, Department of Clinical Laboratory Science, 65 Bergen Street, Newark NJ 07107. (973) 972-5578, (973) 972-8527 (fax), joskotda@umdnj.edu
Connie Mahon MS CLS is the Focus: Bioterrorism guest editor.
Focus Continuing Education Credit: see pages 40 to 42 for learning objectives, questions, and application form.
Copyright American Society for Clinical Laboratory Science Winter 2004
Provided by ProQuest Information and Learning Company. All rights Reserved