Study objectives: Our aim was to clarify the hypothesis that the postsurgical prognosis of patients with non-small cell lung cancer (NSCLC) proven by preoperative diagnostic transbronchial biopsy (TBLB) was worse than that of the patients with NSCLC determined at the time of surgery.
Design: We entered the propensity score as a continuous variable in the Cox proportional hazards model, along with the success/failure of TBLB and other covariates that were adjusted for the bias inherent to the success/failure of the TBLB examination.
Patients: Five hundred ninety-nine consecutive patients with NSCLC undergoing complete resection were divided into two groups. Pathologic diagnosis by TBLB was preoperatively determined in patients belonging to group 1 (n = 367). TBLB was unsuccessful and exploratory thoracotomy or thoracoscopy was followed by surgical resection in patients belonging to group 2 (n = 232). The overall recurrence-free survival rate was examined as the surgical outcomes.
Results: The postsurgical recurrence-free rate was significantly higher in group 2 than in group 1. Group 2 patients showed better prognosis than group 1 patients, even when the data between the two groups were adjusted by propensity score. When the groups were subdivided by the pathologic stage of disease, the subgroup consisting of group 2 patients with stage IA and IB lung cancer still showed a higher recurrence-free rate than those in group 1 by propensity score analysis.
Conclusions: The postsurgical prognosis of the patients with NSCLC was significantly better if the preoperative TBLB was unsuccessful. This result suggested that advanced NSCLC had a tendency to be diagnosed with TBLB and, possibly, that the TBLB procedure might worsen the prognosis of patients with resectable NSCLC. We suggest that intraoperative diagnosis followed by the consecutive resection of NSCLC may be beneficial for improving the surgical outcomes of NSCLC patients.
Key words: bronchoscopy; lung biopsy; lung cancer; statistics; thoracic surgery
Abbreviations: HR = hazard ratio; NSCLC = non-small cell lung cancer; PNB = percutaneous needle biopsy; pStage = pathologic stage; TBLB = transbronchial lung biopsy
**********
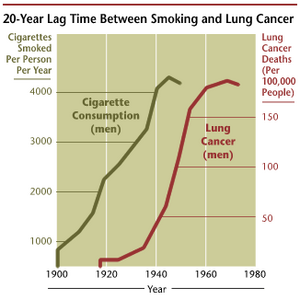
Lung cancer is presently the most common cause of cancer mortality in Japan as well as in the United States. To date, surgical resection for early-stage non-small cell lung cancer (NSCLC) has been the only way to achieve a complete cure. Improving the survival rate of patients undergoing surgical treatment for lung cancer can potentially be achieved by evaluating all perioperative procedures and treatments.
Bronchoscopy has been routinely performed for diagnosing primary lung cancer. Transbronchial lung biopsy (TBLB) is one of the most important examinations to determine the pathologic diagnosis of an indeterminate pulmonary nodule that is suspected to be lung cancer. However, this procedure disrupts vascular and lymphatic structures of the bronchi and alveoli, and might disseminate tumor cells because a part of the pulmonary nodule is torn off by the forceps passing through a channel of the bronchoscope even under fluoroscopic observation. Tumor implantation of the biopsy tract has been reported in the cases of patients undergoing percutaneous needle biopsy (PNB).
We have previously analyzed and reported the influence of TBLB on late outcome after surgical resection retrospectively. (1) We showed that the postsurgical prognosis of the patients with NSCLC that had been diagnosed pathologically with TBLB was worse than that of the patients with all indeterminate pulmonary tumor that had been proven to be NSCLC intraoperatively. Our results also suggested that the prognosis of NSCLC, especially that appearing as an indeterminate nodule or mass in the lung periphery without lymphadenopathy, might be improved if the tumor was surgically resected without a preoperative histopathologic examination.
Then we hypothesized that pathologic examination by intraoperative excisional biopsy via thoracoscopy, rather than preoperative bronchoscopy, followed by curative surgery when the histopathologic diagnosis was a NSCLC, might be beneficial for patients with early lung cancer.
In order to prove this hypothesis from our retrospective analysis, we extended the follow-up period and added the cases concerned. Then we entered the propensity score (2) as a continuous variable in the Cox proportional hazards model, (3) along with the success/failure ratio of TBLB and other covariates that were adjusted for the bias inherent to the success/failure of TBLB examination.
MATERIALS AND METHODS
Eligibility of Patients
Seven hundred eighty consecutive patients with documented primary lung cancer who underwent surgical treatment and preoperative TBLB in our institute between 1982 and 2003 were evaluated retrospectively. The inclusion criteria for entry into the study population included pathologic stage (pStage) IA, IB, IIA, IIB, IIIA, and IIIB NSCLC by the International System of Staging for Lung Cancer. (4) Patients lacking a TBLB record were excluded from the analysis. Patients with small cell carcinoma or neoplasms with a low grade of malignancy, such as adenoid cystic carcinoma or carcinoid tumor, were excluded. Patients with pStage IV NSCLC were excluded from the study. Patients undergoing incomplete resection of the cancer or exploratory thoracotomy were excluded from the analysis. Patients who died of surgical complications were also excluded. A total of 599 patients satisfied the eligibility criteria.
Each case was reviewed by a pathologist to ascertain tumor size, pathologic type, the presence of visceral pleural involvement, lymphatic or vascular invasion, the existence of intrapulmonary metastasis in the resected lung, and involvement of the hilar and mediastinal lymph nodes. Squamons cell carcinoma, adenocarcinoma, adenosquamous cell carcinoma, and large cell carcinoma variants were included in this analysis. Gender and age were also analyzed as the prognostic factors.
TBLB was performed in all patients in a manner already documented elsewhere, (1) except for those patients who refused to undergo bronchoscopy or those who had a small lung cancer < 10 mm in diameter that was located in the periphery of the lung. Briefly, flexible fiberoptic bronchoscopes (Olympus; Tokyo, Japan) were used for performing TBLB. The trachea and all segments of the bronchial tree were visualized through the bronchoscope under superficial local anesthesia. The absence of endobronchial abnormalities was confirmed, and the bronchoscope was then advanced to the lobe and segment known to be the location of the lesion with or without fluoroscopic guidance. A portion of the tumor was grasped and withdrawn using biopsy forceps, and the specimen was sent for histopathologic examination. Transbronchial brushing was also routinely performed for cytologic diagnosis.
Grouping
The cases were divided into two groups. Group 1 included cases of NSCLC diagnosed histopathologically by TBLB. Group 2 included eases of NSCLC without a preoperative pathologic diagnosis even though bronchoscopy had been performed. The pathologic diagnosis of NSCLC in the group 2 patients was determined intraoperatively with needle or excisional biopsy of the tumor through open thoracotomy or thoracoscopy, followed by complete surgical resection.
Surgical Procedure
Patients underwent radical operation (ie, lobectomy, tonsillectomy, or pneumonectomy) with hilar and mediastinal lymph nodal dissection for complete resection of the lung cancer. Pulmonary segmentectomy, wedge resection, and other limited operations were performed in some patients with impaired cardiopulmonary function or whose general condition was poor.
Pastoperative Follow-up
Patients visited the outpatient clinic of our hospital every 3 to 6 months. A chest roentgenogram, CT scan, serum tumor marker measurements, whole-body CT scan, and physical examination were performed to detect lung cancer recurrences. The observation period was terminated on December 31, 2004.
Statistical Analysis
The present study focused on two outcome variables (ie, overall survival and recurrence-free survival). The log-rank test and Cox proportional hazards model (3) were used to examine the relationship between recurrence/survival and various potential prognostic factors individually. The latter included age, gender, extent of surgical resection, usage of thoracoscopy during surgery, pStage, involvement of vascular or lymphatic vessels, pleural involvement, intrapulmonary metastasis, diameter of the main tumor, pathologic type of the cancer, location of the main tumor, and the TBLB yield. Recurrence-free survival time was defined as the time between surgery, and the last follow-up or recurrence of the cancer. If a patient died without cancer recurrence, the patient's survival time was censored at the time of death.
To adjust for the bias inherent to the success/failure of the TBLB examination, propensity scores were used. Propensity analysis aims to identify patients with similar probabilities of successful TBLB on the basis of observed clinical characteristics. (2) With the use of a multivariable logistic regression model that includes basic risk parameters as the independent variables, the probability of a patient's being assigned to the successful TBLB group was determined. The goodness of fit of the propensity score model was obtained by e statistics, and the balancing properties between group 1 and group 2 were satisfied.
The variables included in the propensity score model were age, sex, pathologic type and stage of the lung cancer, pStage, extent of resection, vascular or pleural involvement of the cancer, diameter of the main tumor, and usage of thoracoscopy during surgery. The population was then divided into quintiles according to the propensity score. Within each quintile, the mean propensity scores of group 1 and group 2 were compared, as were their clinical and procedural characteristics.
To adjust for the heterogeneity between the two groups, the propensity score was then entered as a continuous variable in the Cox proportional hazards model, along with the success/failure of TBLB and other covariates listed in Table 1. The validity of the proportional hazards assumption was tested by examining Schoenfeld residuals. All statistical analyses were performed using a statistical software package (STATA, version 8.2; StataCorp; College Station, TX). (5)
We performed [chi square] tests for the bivariate analysis of categoric data. Survival estimates were calculated by the Kaplan-Meier method. Cox proportional hazard modeling was used for unadjusted and adjusted survival analysis.
Prior to the study, the research review board in the department examined and approved our research protocol in light of the Declaration of Helsinki. Informed consent also was obtained from patients being enrolled in the study after the study was explained to them.
RESULTS
Characteristics of Patients
Of the 780 patients registered, we excluded 34 patients with an incompletely documented record of TBLB or postoperative follow-up. We excluded 40 with small cell lung cancer or low-grade malignant lung neoplasms. Sixty patients were excluded because they had pStage IV NSCLC, mainly due to the existence of intrapulmonary metastasis. Eleven patients were excluded because they had undergone incomplete resection of the lung cancer. Twenty-one patients were excluded because they died of postsurgical complications. Thus, 599 patients were eligible for this study. We divided these patients into the following two groups: group 1 included patients with a preoperative histopathologic or cytological diagnosis that had been determined by TBLB or cytology; group 2 included those patients without a preoperative histopathologic or cytological diagnosis. Patients in group 2 received histopathologic diagnoses by intraoperative biopsy through open thoracotomy or thoracoscopy. Three hundred sixty-seven patients were allocated to group 1, and 232 patients were allocated to group 2. The mean ([+ or -] SD) postsurgical observation time was 54.3 [+ or -] 46.3 months in group 1, and 53.2 [+ or -] 40.0 months in group 2.
Table 1 shows the distribution of the clinical and the pathologic risk factors in the two groups of patients, divided into three subgroups by pStage. Younger patients, standard surgery with lobectomy and lymphadenectomy without thoracoscopic examination, squamous cell carcinoma, larger diameter of the tumor, vascular involvement, and advanced pStage were more frequently observed in patients in group 1 (ie, the successful TBLB group).
During the observation period, lung cancer recurrence was identified in 173 patients in group 1 and 49 in group 2. One hundred seventy-eight patients in group 1 and 67 patients in group 2 died during the observation period.
Figure 1 gives Kaplan-Meier estimates of the recurrence-free survival rates of each subgroup. The recurrence-free rates of patients in groups 1 and 2 were examined in each pStage. There was a significant difference between the two subgroups with pStage IA and IB with regard to the yield of the TBLB. The survival rate and the recurrence-free rate were lower in the successful TBLB group.
[FIGURE 1 OMITTED]
In calculating hazard ratios (HRs) regarding disease recurrence in patients whose TBLB examinations were successful (ie, group 1), (1) unadjusted HR[s.sub]2, HRs adjusted only for propensity score,a HRs adjusted only for covariates (not including propensity score) using a stepwise selection method of independent variables at p = 0.0,5 were calculated. When TBLB was not selected at p = 0.0.5, it was forcefully entered in the model to calculate its HRs, while other variables were selected by stepwise selection.
Table 2 shows HRs for recurrence for patients in each pStage calculated by Cox proportional hazard regression model. A Cox proportional hazard model revealed a significant influence of the success of the TBLB on each subgroup. When the data were adjusted for propensity score, a significant difference between the two subgroups consisting of the pStage IA and IB cases was still observed.
DISCUSSION
Our study showed that a positive yield of TBLB was associated with the higher risk of cancer recurrence after the surgical treatment of the patients with resectable NSCLC. TBLB was statistically proved to be one of the independent factors influencing the recurrence-free rate of patients with NSCLC. Furthermore, even if the data were adjusted between the two groups by the propensity score method, we could still observe the difference in the recurrence-free rate between the patients in the two subgroups with pStage IA and IB NSCLC.
We also analyzed the influence of TBLB yield on overall survival rates. A Kaplan-Meier analysis showed a significantly negative impact on the survival rate. However, when the data were adjusted for propensity score, no significant difference in the survival rates was found between the two groups. This result might be attributed to the fact that some patients died of nonneoplastic diseases.
In the analysis, pStage was entered as a continuous variable. When it was used as a categoric variable, the results were not significantly changed. Furthermore, an analysis that stratified the subjects by pStage produced results similar to those reported herein, revealing the robustness of our results.
TBLB, in addition to PNB, has been most commonly used to determine the diagnosis of intrapulmonary nodules or masses that were suspected of being lung cancer. A histopathologic diagnosis can be established in 50 to 70% of patients with peripheral pulmonary masses or nodules in whom biopsy specimens were obtained through a bronchoscope. (6-8) However, there is still controversy over performing preoperative pathologic diagnosing procedures.
Preoperative diagnosis by TBLB or PNB may avoid exploratory surgery for the purpose of lung biopsy. This is beneficial for patients with malignant lung neoplasms that have not been indicated for surgical therapy, such as small cell lung cancer or far advanced NSCLC, if they are properly diagnosed.
However, the diagnosis of benign lung diseases is usually difficult to achieve with TBLB or PNB. Charig et al (9) suggested that, if a solitary pulmonary nodule had a high clinical suspicion of malignancy and the patient was a candidate for surgery, PNB was of limited value. TBLB through bronchoscopy may be a good approach for obtaining a tissue diagnosis in large, central lung masses or in those with endobronchial lesions, as the diagnostic yield is 70% and 90%, respectively. (10-14) However, for the patient with a peripheral lung nodule, there is less of a role for bronchoscopy. (11) The bronchoscopic diagnostic yield is proportional to the size of the lung lesion. The sensitivity for peripheral lesions < 2 cm in diameter was 0.33 from a metaanalysis by Schreiber and McCrory. (15) In the evaluation of the small solitary pulmonary nodule, TBLB has been shown to provide no measurable preoperative benefit to the patient, as it does not obviate the need for surgery. (14) Torrington and Kern (14) have suggested that no change in diagnosing strategy might occur for patients with an indeterminate pulmonary nodule that was suspected of being a resectable NSCLC by chest CT scan.
During TBLB or brushing, the neoplastic tissue is bluntly torn from the main tumor. This procedure is just like incisional biopsy, a partial surgical resection of the tumor that is performed for diagnostic purposes. The possibility of implanting tumor cells via the biopsy tract cannot be ignored in TBLB.
Some studies (16) have described the extremely rare possibility of tumor seeding during biopsy procedures, as 1 in 4,000 transthoracic needle biopsies. However, Berger et al (17) suggested that the performance of PNB is not justified in patients with potentially operable malignancies, owing to the risk of tumor cell dissemination.
In some publications, it has been stated that the performance of incisional biopsy to determine the preoperative diagnosis of a malignant neoplasm has adverse effects on the postsurgical outcome. The recurrence-free survival rates after one-stage mastectomy and partial axillary dissection in breast cancer patients with invasive ductal carcinoma were significantly lower in those patients with residual cancer tissue after undergoing a preoperative biopsy. (18) In patients with malignant melanoma, those undergoing incisional biopsy developed distant metastasis more frequently than did those undergoing excisional biopsy. (19) In patients with nasopharyngeal carcinoma, it has been reported (20) that patients undergoing radiotherapy within 14 days after biopsy showed a significantly higher 5-year survival rate (61%) than those undergoing radiotherapy beyond 14 days. Controversial conclusions have, however, been drawn. In patients with malignant melanoma, incisional biopsy did not adversely affect prognosis in terms of either local recurrence or mortality. (21)
No evidence of tumor implantation in the bronchial lumen caused by TBLB has been obtained, to date. However, the local recurrence of lung cancer in an airway cannot be distinguished from the iatrogenic spread of the neoplasm. It is thus understood that the dissemination might occur via lacerated arterioles, venules, lymphatics, and/or airway tracts.
In our study, the pathologic characteristics of patients in group 1 (ie, those who underwent successful TBLB) were significantly different from those in group 2 (ie, those who underwent unsuccessful TBLB). The main tumor diameter was significantly larger in group 1. Thus, advanced lung cancer tends to be diagnosed by TBLB. This result is attributable to the fact that the rate of diagnostic yield by bronchoscopy is related to the pathologic nature of the lesion. Diagnosis by TBLB was more frequent in lesions with fuzzy borders, larger diameters, and/or central fibrosis. (22,23) When lesions were located in the inner two thirds of the lung, the diagnostic yield was higher than that for lesions located in the peripheral third of the lung. (24)
However, in our study, after adjustment by the propensity score method, we still observed the statistically significant differences in prognosis between groups 1 and 2. It is thus suggested that the transbronchial procedure might worsen the prognosis of patients with resectable NSCLC.
Thoracoscopic biopsy of an indeterminate pulmonary nodule has been applied for the pathologic diagnosis of lung neoplasms located in the periphery. Nodules or masses located in the deep lung parenchyma could even be biopsied via thoracoscopy with preoperative CT-guided marking. In our institution, thoracoscopic biopsy has been performed for indeterminate peripheral pulmonary nodules with unsuccessful preoperative transbronchial biopsy since 1993. Radical resections through open thoracotomy were subsequently performed. No complications associated with the thoracoscopic procedure have been observed to date.
Thoracoscopic lung biopsy is a minimally invasive surgical procedure. It can be followed by radical resection if the pathologic diagnosis is NSCLC. Mitruka and colleagues (25) described the safety and accuracy of thoracoscopic excisional biopsy for peripheral lung nodules, comparing it to CT scan-guided needle biopsy.
Our results showed that NSCLC with poorer prognosis had a tendency to be easily diagnosed with TBLB. Our results also suggested that the prognosis of NSCLC, especially that appearing as an indeterminate nodule or mass in the lung periphery without lymphadenopathy, might be improved if the tumor is surgically resected without preoperative histopathologic examination. We recommend performing a prospective randomized study to reveal the effect of preoperative TBLB on the postsurgical prognosis for patients with NSCLC. We suggest that a pathologic examination without preoperative bronchoscopy, but through an intraoperative excisional biopsy via thoracoscopy, followed by curative surgery when the histopathologic diagnosis is NSCLC, might be beneficial for patients with early-stage lung cancer.
REFERENCES
(1) Nakajima J, Takamoto S, Matsumoto J, et al. Effect of preoperative transbronchial biopsy on prognosis of non-small cell lung cancer. Asian Cardiovasc Thorac Ann 2004; 12:330-335
(2) Rosenbaum PR. Observational studies. 2nd ed. New York, NY: Springer, 2002; 296-302
(3) Cox DR. Regression models and life tables. J R Stat Soc 1972; 34:187-220
(4) Mountain CF. Revisions in the international system for staging lung cancer. Chest 1997; 111:1710-1717
(5) StataCorp. STATA for Windows: version 8.2. College Station, TX: StataCorp, 2004
(6) Gasparini S, Ferretti M, Secchi EB, et al. Integration of transbronchial and percutaneous approach in the diagnosis of peripheral pulmonary nodules or masses: experience with 1,027 consecutive cases. Chest 1995; 108:131-137
(7) Chechani V. Bronchoscopic diagnosis of solitary pulmonary nodules and lung masses in the absence of endobronchial abnormality. Chest 1996; 109:620-625
(8) Reichenberger F, Weber J, Tamm M, et al. The value of transbronchial needle aspiration in the diagnosis of peripheral pulmonary lesions. Chest 1999; 116:704-708
(9) Charig MJ, Stutley JE, Padley SPG, et al. The value of negative needle biopsy in suspected operable lung cancer. Clin Radiol 1991; 1991; 44:147-149
(10) Jett J, Feins R, Kvale P, et al. Pretreatment evaluation of non-small-cell lung cancer: The American Thoracic Society and The European Respiratory Society. Am J Respir Crit Care Med 1997; 156:320-332
(11) Arroliga AC, Matthay RA. The role of bronchoscopy in lung cancer. Clin Chest Med 1993; 14:87-98
(12) Stringfield JT, Markowitz DJ, Bentz RR, et al. The effect of tumor size and location on diagnosis by fiberoptic bronchoscopy. Chest 1977; 72:474-476
(13) Baaklini WA, Reinoso MA, Gorin AB, et al. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest 2000; 117:1049-1054
(14) Torrington KG, Kern JD. The utility of fiberoptic bronchoscopy in the evaluation of solitary pulmonary nodules. Chest 1993; 104:1021-1024
(15) Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest 2003, 123(suppl): 115S-128S
(16) Nordenstrom B, Bjork VO. Dissemination of cancer cells by needle biopsy of lung. J Thorac Cardiovasc Surg 1973; 65:671
(17) Berger RL, Dargan EL, Huang BL. Dissemination of cancer cells by needle biopsy of the lung. J Thorac Cardiovasc Surg 1972; 63:430-432
(18) Watt-Boolsen S, Ottesen G, Andersen JA, et al. Significance of incisional biopsy in breast carcinoma: results from a clinical trial with intended excisional biopsy. Eur J Surg Oncol 1989; 15:33-37
(19) Austin JR, Byers RM, Brown WD, et al. Influence of biopsy on the prognosis of cutaneous melanoma of the head and neck. Head Neck 1996; 18:107-117
(20) Cai WM, Zhang HX, Hu YH, et al. Influence of biopsy on the prognosis of nasopharyngeal carcinoma: a critical study of biopsy from the nasopharynx and cervical lymph node of 649 patients. Int J Radiat Oncol Biol Plays 1983; 9:1439-1444
(21) Lees VC, Briggs JC. Effect of initial biopsy procedure on prognosis in Stage I invasive cutaneous malignant melanoma: review of 1086 patients. Br J Surg 1991; 78:1108-1110
(22) Chechani V. Bronchoscopic diagnosis of solitary pulmonary nodules and lung masses in the absence of endobronchial abnormality. Chest 1996; 109:620-625
(23) Nomori H, Horio H, Fuyuno G, et al. Lung adenocarcinomas diagnosed by open lung or thoracoscopic vs bronchoscopic biopsy. Chest 1998; 114:40-44
(24) Baaklini WA, Reinoso MA, Gorin AB, et al. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest 2000; 117:1049-1054
(25) Mitruka S, Landreneau RJ, Mack MJ, et al. Diagnosing the indeterminate pulmonary nodule: percutaneous biopsy versus thoracoscopy. Surgery 1995; 118:676-684
* From the Departments of Cardiothoracic Surgery (Drs. Nakajima and Takamoto) and Public Health (Dr. Sato), Graduate School of Medicine, University of Tokyo, Tokyo, Japan.
Manuscript received April 29, 2005; revision accepted June 14, 2005.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal. org/misc/reprints.shtml).
Correspondence to Jun Nakajima, MD, PhD, Department of Cardiothoracic Surgery, Graduate School of Medicine, 7-3-1, Hongo, Bunkyo-ku, University of Tokyo, Tokyo, Japan 113-8655; e-mail: nakajima-tho@h.u-tokyo.ac.jp
COPYRIGHT 2005 American College of Chest Physicians
COPYRIGHT 2005 Gale Group