Novafed
Pseudoephedrine is a sympathomimetic amine commonly used as a decongestant. The salts pseudoephedrine hydrochloride and pseudoephedrine sulfate are found in many over-the-counter preparations either as single-ingredient preparations, or more commonly in combination with antihistamines and/or paracetamol/ibuprofen. more...
This agent is often referred to by consumers as Sudafed, which is the trademark for a common brand of pseudoephedrine hydrochloride. Other brand names include Afrinol, Novafed, and Cenafed.
Unlike antihistamines, which modify the systemic histamine-mediated allergic response, pseudoephedrine only serves to relieve nasal congestion commonly associated with colds or allergies. The advantage of oral pseudoephedrine over topical nasal preparations, such as oxymetazoline, is that it does not cause rebound congestion (rhinitis medicamentosa). However, the disadvantage of oral pseudoephedrine is that it can cause high blood pressure.
Nomenclature
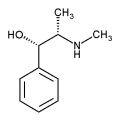
Pseudoephedrine is the International Nonproprietary Name (INN) of the (1S,2S)- diastereomer of ephedrine (which has 1R,2S- configuration). Equivalent names are (+)-pseudoephedrine and D-pseudoephedrine. (Reynolds, 1989)
(-)-Pseudoephedrine or L-pseudoephedrine then designates the enantiomer of pseudoephedrine.
Chemistry
Pseudoephedrine, a phenethylamine, is a structural isomer of the popular weightloss/energy supplement and asthma medication, ephedrine. Ephedrine is an alkaloid extracted from the Ephedra plant, which produces it naturally as a racemic mixture. That is, ephedrine molecules occur as two "mirror images," inasmuch as a pair of hands do (See the article on entantiomers). The pharmacologic properties of each "reflection" often share similarities and differences. The (-) or levorotatory isomer is a very potent sympathomimetic amine and anorectic, thus responsible for the amphetamine-like stimulation that is characteristic of Ephedra products. The (+) or dextrorotatoryisomer, aka pseudoephedrine, is far less potent as a stimulant. However, it retains much of ephedrine's ability to open airways and nasal passages.
Mode of action
Pseudoephedrine is a sympathomimetic amine - that is, its principal mechanism of action relies on its indirect action on the adrenergic receptor system. Whilst it may have weak agonist activity at α- and β-adrenergic receptors, the principal mechanism is to displace noradrenaline from storage vesicles in presynaptic neurons. The displaced noradrenaline is released into the neuronal synapse where it is free to activate the aforementioned postsynaptic adrenergic receptors.
The vasoconstriction that pseudoephedrine produces is believed to be principally an α-adrenergic receptor response. Whilst all sympathomimetic amines, to some extent, have decongestant action; pseudoephedrine shows greater selectivity for the nasal mucosa and a lower affinity for central nervous system (CNS) adrenergic-receptors than other sympathomimetic amines. (+)-(1S,2S)-pseudoephedrine shows far lower CNS activity than other Ephedra alkaloids, ephedrine, and (-)-(1R,2R)-pseudoephedrine.
Read more at Wikipedia.org