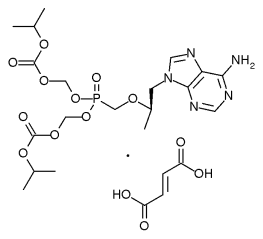
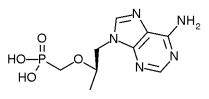
Viread
Tenofovir disoproxil fumarate (TDF), with the trade name Viread®, belongs to a class of antiretroviral drugs known as nucleotide analogue reverse transcriptase inhibitors (NtRTIs), which block reverse transcriptase, an enzyme crucial to viral production in HIV-infected people. more...
Tenofovir is marketed by Gilead Sciences with the brand name Viread®. It is also available in a fixed-dose combination with emtricitabine in a product with the brand name Truvada® for once-a-day dosing. (Emtricitabine is marketed as a single-compound product called Emtriva®, also marketed by Gilead.) A fixed-dose triple combination of tenofovir, emtricitabine and efavirenz (Sustiva®, marketed by Bristol-Myers Squibb) is in development.
Tenofovir is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in adults. This indication is based on analyses of plasma HIV-1 RNA levels and CD4 cell counts in controlled studies of tenofovir in treatment-naïve and treatment-experienced adults. There are no study results demonstrating the effect of tenofovir on clinical progression of HIV.
History
Tenofovir was discovered through a collaborative research effort between Dr Antonin Holy at the Academy of Sciences of the Czech Republic (IOCB) in Prague, and Dr Erik DeClercq, Rega Institute for Medical Research, Katholic University, Belgium.
Tenofovir was approved by the U.S. Food and Drug Administration (FDA) on October 26, 2001 and is marketed by Gilead Sciences. It is currently in late-stage clinical trials for the treatment of hepatitis B.
The fixed-dose combination of tenofovir with emtricitabine was approved on 2004-08-02 for once-a-day dosing. The fixed-dose triple combination of tenofovir, emtricitabine and efavirenz is in development.
Read more at Wikipedia.org