Although acute myocardial lesions have been described pathologically in as many as 85 percent of patients with valvular infective endocarditis, [1] mural endocarditis in which the infective process is cofined to the nonvalvular endocardium is exceedingly rate, with only 22 reported cases through 1978. [2] Most fo these patients were immunosuppressed or otherwise debilitated and severely ill. A recent review [3] highlighted the tendency for peripheral emboli commonly to occur when mitral valve endocarditis is complicated by a left atrial mural vegetation. Indeed, patients with left atrial mural vegetation may define a subpopulation at increased risk of embolization.
CASE REPORT
A 45-year-old woman was hospitalized at another institution because of fever and confusion. She was well until two weeks previously when she noted fever to 38.5[degrees]C, malaise and a skin rash described as a generalized vesicular eruption by her physician. One week prior to entry, she developed rigors, fevers to 40[degrees]C, muscular aches and joint pains. Penicillin was prescribed and though her rash resolved, she began to have profuse watery diarrhea. The day of admission the patient's husband found her to be confused and took her to the emergency room. There was no history of murmur, congenital heart disease, dental work or intravenous drug abuse. The patient had two children and she worked as a self-employed housecleaner. She had no significant past medical history. Family history was nmegative for cardiorespiratory disease.
Physical examination revealed a middle-aged woman who appeared dehydrated. The pulse was 108 beats per minute with the patient in the supine position, which increased to 120 beats per minute while sitting, whereas the blood pressure fell from 96/0 to 80/0 mm Hg. Temperature was 38.7[degrees]C. Head and neck examination showed conjunctivitis with petechiae on her eyelids, upper palate and buccal mucosa. The neck was supple and there was no lymphadenopathy. There are no jaundice, clubbing or Roth spots. Chest examination was normal. Cardiovascular examination revealed a jugular venous pressure of 3 cm above the sternal angle, normal carotid artery pulsations and a normal apical impulse. The heart sound were normal and no extra sounds were noted on auscultation. A 1-2/6 pansystolic murmur was heart at the apex which radiated faintly to the axilla. Peripheral pulses were palpable and the abdomen was normal without organomegaly. Splinter hemorrhages and Janeway lesions were noted on the right second and third digits and left thumb. Aside from disorientation, the central nervous system examination was within normal limits, with no focal findings.
Significant initial laboratory investigations were as follows: white blood cell count 11.2; 93 percent granulocytes; hemoglobin, 99 g/L; electrolytes, normal; blood urea, 15.4 mmol/L; creatinine, 230 mmol/L; and urinalysis, 3+ blood and 3+ protein. Prothrombin time was 13.3 s (normal, less than 12.5 s); PTT, 29 s. The DIC screen was negative. Arterial blood gas value analysis and chest x-ray filn were reported as normal. The electrocardiogram revealed sinus tachycardia. The P wave morphology was normal.
The patient was treated with intravenous crystalloid cloxacillin and gentamicin for a presumptive diagnosis of infective endocarditis. Vitamin K was administered. On the second hospital day, blood cultures grew Staphylococcus aureus, which was sensitive to both antimicrobials, and echocardiogram revealed a left atrial mass measuring 2 X 1.5 cm, which was thoought to be a prolapsing left atrial myxoma. Later that day her right forearm and foot became cool and pulseless. Embolectomy of the right brachial and posterior tibial arteries was performed, and pathology revealed septic emboli. An MRI scan of her head showed a 1.5-cm lesion in the left cerebellar hemisphere consistent with a septic embolus. Smaller lesions were seen in the internal capsules bilaterally.
Upon transfer to this institution, she was afebrile and hemodynamically stable. The pulse was 100 beats per minute and blood pressure, 120/80 mm Hg. The physical examination was unchanged. Her coagulopathy had resolved and renal function was improving. Repeat two-dimensional and M-mode echocardiography again demonstrated a freely moving 2.0 X 1.5-cm predunculated mass attached to the posterior mural left atrium and abutting the posterior mitral leaflet. On a color Doppler test, a mitral regurgitant jet struck the posterior left atrial wall in this area. The structure prolapsed into the left ventricle during diastole (Fig 1). Using the technique of Helmche et al, [4] the mitral regurgitation was mild, having a regurgitant jet area to left atrial area ratio of 17 percent.
A median sternotomy was performed on the evening of transfer. Findings at left atriotomy included a normal appearing mitral valve and interatrial septum, with a friable 2-cm mass attached to the posterior wall of the left atrium. This was excised intact (Fig 2), and she recovered without any postoperative complications. Her mental status normalized. She completed a one-month course of intravenously administered cloxacillin and was discharged home on a regimen of orally administered cloxacillin, 500 mg four times a day. A postoperative echocardiogram again demonstrated mild mitral regurgitation with the jet striking the posterior left atrial wall on a color Doppler test.
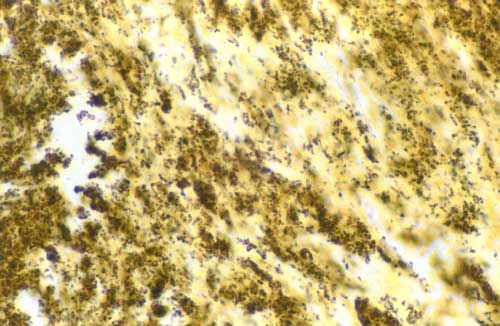
Microscopic examination of the mass showed granulation and necrotic tissue with clusters of Gram-positive cocci suggestive of bacterial colonies (Fig 3). No myxomatous tissue was noted. Scrapings of left atrial endocardium immediately beneath the mass revealed hemorrhagic fibrinous debris with focal clustering of neutrophils and clusters of Gram-positive and poorly stained cocci consistent with infective mural endocarditis. Fungal staining was negative.
DISCUSSION
We present the case of a woman who had previously unrecognized mitral regurgitation. She developed an acute febrile illness and blood cultures were positive for S aureus. A vegetation, which had partially embolized to her upper and lower extremities and brain, was evidnt on echocardiography. At operation, left atrial mural endocarditis with otherwise normal-appearing cardiac anatomy was found.
Previously reported episodes of lone bacterial mural] endocarditis have usually been associated with underlying disease processes raning from thrombophlebitis to bronchiectasis and paralysis agitans. [5] In some of these cases, the pathogenesis of mural involvement has been ascribed to direct extension of myocardial abscesses; however, the etiology in others remained obscure. Mural endocarditis has been reported in the setting of infected mural thrombi or aneurysms, jet lesions from ventricular septal defects and idiopathic hyperthrophic subaortic stenosis. [6] In addition, left atrial mural endocarditis may be acquired from the extension of a pulmonary abscess through a pulmonary vein. [7]
According to recent reviews, fungal endocarditis confined to the mural surface of normal hearts is observed with immunosuppression from either lymphoproliferative disorders and their treatment or organ transplantation immunomodulation. [5,8] We postulate that previously undetected mitral regurgitation may have created a jet lesion on the posterior left atrial wall, creating an anatomic substrate for infection.
Staphylococcus aureus is an unusual pathogen in native valve infective endocarditis, witih only an overall 1.5 to 13 percent prevalence in recent review. [8] This virulent organism usually lodges itself on normal valves and is more frequent in intravenous drug abusers.
M-mode and two-dimensional echocardiography have been of diagnostic and prognostic value in bacterial and fungal endocarditis. [9] The echocardiographic findings in this patient were striking, with the vegetation prolapsing into the left ventricle during diastole, only to recoil into the left atrium during systole, mimicking a myxoma. Although 75 to 80 percent of myxomas are found in the left atrium, they usually are attached to the limbus of the fossa ovalis by a short fibrovascular stalk and only rarely present as extremely fragile papillary excrescences which have a sessile attachment to the interatrial septum or posterior atrial wall. [10] Superinfection and embolism are well recognized complications of atrial myxomas and though our patient did not manifest any evidence of hemodynamic obstruction, the preoperative diagnosis was an infected myxoma of the posterior left atrial wall with emboli.
It is imporant to recognize that the absence of vegetation on two-dimensional echocardiogram does not rule out the diagnosis of endocarditis. Fifteen percent of infective vegetations can be missed by echocardiography [6] and false-negative results up to 50 percent have been reported in a small series of cases of Aspergillus endocarditis. [7]
REFERENCES
[1] Buchbinder N, Roberts W. Left-sided valvular active infective endocarditis. Am J Med 1972; 53:20-35
[2] Walsh TJ, Hutchins GM. Aspergillus mural endocarditis. Am J Clin Pathol 1979; 71:640-44
[3] Kim JH, Wiseman A, Kisslo J, Durack DT. Echocardiographic detection and clinical significance of left atrial vegetations in active infective endocarditis. Am J Cardiol 1989; 64:950-52
[4] Helmche F, Nanda NC, Hsuing MC, Goto B, Adey CK, Goyal RG, et al. Color Doppler assessment of mitral regurgitation with orthogonal planes. Circulation 1987; 75:175-83
[5] Buchbinder N, Roberts A. Active infective endocarditis confined to mural endocardium. Arch Pathol 1972; 93:435-40
[6] Herzog C, Carson P, Michaud L, Asinger R. Two-dimensaional echocardiographic imaging of left ventricular mural vegetations. Am Heart J 1988; 115:684-86
[7] Lang D, Leisen J, Elliot J, Lewis J, Wenott D, Quinn E. Echocardiographically silent Aspergillus mural endocarditis. West J Med 1988; 149:334-38
[8] Milstec M, Berger A. True bacterial mural endocarditis. Chest 1971; 59:103-05
[9] Woods G, Wood R, Shaw B. Aspergillus endocarditis in patients without prior cardiovascular surgery: report of a case in a liver transplant recipient and review. Rev Infect Dis 1989; 2:263-72
[10] Novick RJ, Dobell ARC. Tumors of the heart. In: Hammond GL, ed. Thoracic and cardiovascular surgery, 5th ed. Norwalk, CT: Appleton-Century-Crofts (in press)
COPYRIGHT 1991 American College of Chest Physicians
COPYRIGHT 2004 Gale Group