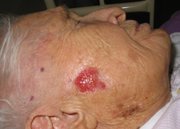
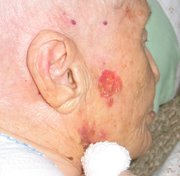
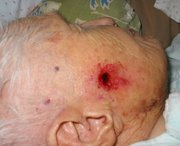
WASHINGTON -- Imiquimod continues to show efficacy in the treatment of superficial basal cell carcinomas, Dr. John K. Geisse reported in a poster session at the annual meeting of the American Academy of Dermatology.
And the results of a multicenter, double-blind trial now have made it clear that a once-daily dosage, 5-7 days per week, is preferable to twice-daily application of the immune modulating cream.
Twice-daily dosages are associated with high rates of skin erosion and other severe adverse reactions. Local reactions such as itching, pain, and erythema in this study were mild to moderate and "well tolerated," said Dr. Geisse of Vallejo, Calif.
The study randomized 128 patients to imiquimod 5% cream twice daily, once daily, 5 times per week, or 3 times per week. A control group received vehicle only. All patients were adults and had biopsy-confirmed primary basal cell carcinomas.
A complete response (defined as no histologic evidence of tumor after 12 weeks of treatment) was seen in 100% of the twice daily group, which included 10 patients. This regimen was discontinued, however, because it was not well tolerated.
A complete response also was seen in 87% of the 31 patients on the once-daily regimen. Among those who used the cream five times per week, 81% of 26 patients had complete responses, as did 52% of the 29 patients applying imiquimod three times each week. A complete response also was seen in 19% of the 32 patients given vehicle only.
A total of 17 patients discontinued treatment because of adverse effects, 4 from the twice-daily group, 9 from the once-daily group, and 4 from the five times per week group.
Imiquimod 5% cream looks "promising" as a medical treatment for superficial basal cell carcinoma, Dr. Geisse concluded, adding that larger clinical studies are planned.
The study was sponsored by 3M Pharmaceuticals.
COPYRIGHT 2001 International Medical News Group
COPYRIGHT 2001 Gale Group