ABSTRACT. Background: Sodium polystyrene sulfonate (Kayexalate) commonly is used in treating hyperkalemia. As a cation exchange resin, it also can be used to reduce the potassium content of enterai nutrition formulas. This study evaluates the use of Kayexalate to reduce potassium in one high-protein enterai formula and describes the quantitative analysis of the product. Methods: Sodium polystyrene sulfonate and enterai formula were mixed into a slurry and allowed to settle, and then the supernatant was decanted off and tested as samples. Three sample concentrations were analyzed: a control not subjected to potassium reduction, 0.5 g of Kayexalate per mEq K+ sample, and a 1 g/mEq K+ sample. Of these samples, moisture, lipid, protein, carbohydrate, ash, and mineral content were obtained. Results: Compared with the control, the percentage decrease of potassium ranged from 25% to 36%, depending on the concentration of Kayexalate. A significant increase of 324% in sodium concentration was found in the 1.0 g/mEq K+ sample. Although there was no change in magnesium content, a slight increase in phosphorus, iron, and zinc was evident. Conclusions: The treatment of an enterai formula with sodium polystyrene sulfonate significantly increases its sodium content, with a modest decrease in potassium content. Clinicians using this method in clinical practice should be aware of the increase in sodium content. (Journal of Parenteral and Enteral Nutrition 28:76-78, 2004)
Sodium polystyrene sulfonate (Kayexalate) is often used to treat non-life-threatening hyperkalemia. It is usually administered orally or rectally to reduce serum potassium concentration. At the University of Minnesota, sodium polystyrene sulfonate has been used to control serum potassium levels by reducing the potassium content of enterai formulas. This technique has been used in enterai feedings of children with renal failure1 and critically ill, enterally fed adult patients. It is particularly appropriate when a hyperkalemic patient is a candidate for a higher protein formula such as Impact 1.5.
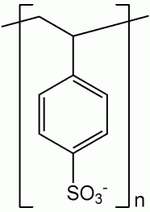
Sodium polystyrene sulfonate is a cation exchange resin that preferentially binds potassium in exchange for sodium (Fig. 1). It has an in vitro exchange capacity of approximately 3.1 mEq (in vivo approximately 1 mEq) of potassium per gram. The sodium content is approximately 100 mg (4.1 mEq) per gram of the drug.2 When sodium polystyrene sulfonate is mixed with an enterai formula, the insoluble resin exchanges sodium for potassium and then settles to the bottom of the container. The potassium-depleted, sodium-enriched formula is decanted and administered to the patient via an appropriate enterai access.
This study was undertaken to determine the nutrient content of a high-protein formula after treatment with sodium polystyrene sulfonate. Although this technique has been used for years in various clinical settings, few studies have been undertaken to evaluate the effect on the nutrient content of treated enterai formulas. Fassinger et al3 reported that treatment of infant formula reduced its potassium content by 62%. However, the effect on a formula designed for critically ill adults could be significantly different.
MATERIALS AND METHODS
Three 250 mL samples of enterai formula composed of Impact 1.5 supplied by Novartis Nutrition Corporation (Saint Louis Park, MN) were chosen for analysis. The control was used to measure the amount of potassium measured as mEq K+/250-mL sample. For the 2 remaining samples, either 0.5 or 1.0 g of sodium polystyrene sulfonate per mEq K+, respectively 5.38 g or 10.75 g, was added to reduce the potassium content. The expected potassium content of untreated samples was based upon Novartis Nutrition's available literature. Initially, the experimental samples were prepared by mixing the enterai formulas with Kayexalate for at least 20 minutes and allowing the mixture to settle out over 24 hours. Only the supernatant, decanted away from the settled sodium polystyrene sulfonate particulate, was tested for moisture, carbohydrate, fat, protein, and mineral composition.
For moisture content, the samples were heated to 70°C under vacuum. Nonvolatile physical dispersant was used to break up large aggregates of dried material. The lipids were isolated in hydrochloric acid and extracted with petroleum ether. The sample then was burned in an 800°C furnace under an oxygen atmosphere. The aliquot passed through a reduction tube and sorption agents to remove carbon dioxide. The nitrogen content of the gas was then measured in a thermal conductivity cell and validated against an external standard.
The organic portion of the sample was vaporized, and the residual ash was weighed for calculation of the carbohydrate content. The sample, now free of organic material, was dissolved in hydrochloric acid for measurement of calcium, copper, iron, magnesium, potassium, sodium, and phosphorus. An inductively coupled plasma optical emission spectrophotometer allowed measurement of the minerals against a reference standard.
RESULTS
Changes occurred in the potassium, sodium, calcium, phosphorus, and protein content after treatment with Kayexalate (Table I). Potassium decreased by 25% with 0.5 g/mEq K+ and by 36% with 1.0 g/mEq K+. Calcium was reduced by approximately 14% in both samples. Sodium, on the other hand, increased by 243% over baseline in the solution containing 0.5 g/mEq K+ and 342% over baseline in the solution containing 1.0 g/mEq K+. Less dramatic changes occurred to decrease protein and increase phosphorus content.
DISCUSSION
Hyperkalemia most frequently occurs in the renal failure patient who needs a low-protein, high-carbohydrate diet. However, at the University of Minnesota, a major referral center for liver failure and transplantation, hyperkalemia occasionally occurs. Typically, these patients are in the critically ill pretransplant intensive care unit setting. Severe metabolic encephalopathy in liver failure necessitates intubation and enterai feeding with a low-carbohydrate-high-protein diet to maximize visceral protein stores. Liver failure patients are often malnourished, and enterai feeding is our preferred choice with a functional gastrointestinal tract.
Supplemental enterai formulas such as Impact 1.5 have been found to improve outcomes in critically ill surgical patients and have been used successfully in critically ill transplant patients at the University of Minnesota. Although other enterai nutrition formulations may be lower in potassium, they do not offer the immunoenhancing clinical benefits that Impact 1.5 provides.4 A critically ill pretransplant patient with an actual weight of 90 kg, a dry weight of 60 kg, and hyperkalemia would be a candidate for enterai support with Impact 1.5. After treatment with Kayexalate, 1000 mL of Impact 1.5 would supply 1500 calories (25/kg dry weight), 74 g of protein (1.2 g/kg dry weight), and only 700 mL free water. This treated formula contains 26 mEq potassium and 185 mEq sodium. For comparison, an untreated Impact 1.5 formula would have 43 mEq potassium and 57 mEq sodium. It is important for the clinician to evaluate each patient individually, and there may be critical situations where the increased sodium content of Kayexalatetreated formulas would be a disadvantage in fluid and sodium restricted patients.
CONCLUSIONS
The resin's cation exchange performs the same function when treating formulas as it does in the gastrointestinal tract when administered orally or rectally. just as serum sodium levels are expected to be elevated after Kayexalate's administration,5 the formula's sodium content is understandably increased. When using this method to reduce potassium load, the clinician should expect that IV fluid management will have to take into account the 2- to 3-fold increase in the formula's sodium load. Calculation of any nitrogen balance studies should also be adjusted.
Kayexalate-treated formulas add another option for delivering enterai support. Because higher-protein formulas have correspondingly higher potassium content, providing an optimal level of nutrition support via the gastrointestinal tract to a highly stressed hyperkalemic liver-failure patient can be challenging. This approach allows more flexibility in managing electrolytes in critically ill patients.
ACKNOWLEDGMENTS
The authors graciously thank Novartis Nutrition (Sandoz) and Ann Erickson, RD, for her technical and analytical assistance.
REFERENCES
1. Bunchman TE, Wood EG, Schenck MH, Weaver KA, Klein BL, Lynch RE. Pretreatment of formula with sodium polystyrene sulfonate to reduce dietary potassium intake. Pediatr Nephrol. 1991;5:29-32.
2. Novartis Nutrition product literature, Available at: http:// www.novartisnutrition.com/us/productDetail?id = 38&source = summary. Accessed May 19, 2003.
3. Fassinger N, Dabbagh S, Mukhopadhyay S, Lee DY. Mineral content of infant formula after treatment with sodium polystyrene sulfonate. Adv Pent Dial. 1998;14:274-277.
4. Beale RJ, Bryg DJ, Bihari DJ. Clinical effects of enterai immunonutrition on intensive care patients: a meta-analysis. Crit Care Med. 1999;27:2799-2805.
5. Product literature. Sanofl-Synthelabo Inc., NY, NY.
Andrew L. Rivard, MD; Sandra M. Raup, RD; and Gregory J. Beilman, MD
Department of Surgery, University of Minnesota Medical School, Minneapolis, Minnesota
Received for publication November 1, 2002.
Accepted for publication October 15, 2003.
Correspondence: Greg J. Beilman, MD, Associate Professor of Surgery and Anesthesiology Director, Surgical Critical Care Department of Surgery, University of Minnesota Medical School, Mayo Mail Code 11, 406 Harvard Street S.E., Minneapolis, MN 55455. Electronic mail may be sent to beilm001@umn.edu.
Copyright American Society for Parenteral and Enteral Nutrition Mar/Apr 2004
Provided by ProQuest Information and Learning Company. All rights Reserved