AUSTRALIANS love their Pharmaceutical Benefits Scheme (PBS). It keeps medicines affordable and controls health costs. But it is loathed by the giant pharmaceutical companies. Not only because it limits their profits in Australia, but also because it is being seen as a model that might be applied elsewhere around the world.
How does the PBS work? Drug companies charge high prices for new medicines because they have exclusive patent rights for 20 years. Under the PBS, the Australian Government uses its buying power to negotiate lower prices in return for drugs being listed for public subsidy and therefore being more likely to be prescribed by doctors.
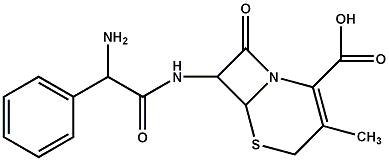
The PBS has an advisory committee which compares the price and effectiveness of new drugs with the prices of similar generic medicines whose patents have expired. This 'reference pricing' works: the wholesale prices of common prescription medicines are three to ten times lower in Australia than in the US. For example, last year the wholesale price of 500 mg of the antibiotic Keflex (cephalexin) was $5.22 while the US price was $89.83. The wholesale price of 20 mg of Nolvadex (tamoxifen) used for breast cancer treatment was $51.36 in Australia but $150.74 in the US. (1)
The PBS makes commonly prescribed medicines available to all Australians at subsidized prices--from $14 to $22 for wage earners and from $2.15 to $3.60 for pensioners. The difference between the wholesale price and the subsidized price is the cost to taxpayers. The PBS only lists new drugs if they offer real health benefits and value for money.
The US, prompted by drug companies, declared from the beginning of the recent free trade negotiations that 'reference pricing' was on the table. Canberra denied that the PBS was up for grabs. But health experts believe last-minute concessions will lead to higher prices:
[ILLUSTRATION OMITTED]
* Drug companies have more opportunity to influence the advisory committee before decisions are made. The deal also sets up an independent process to review decisions not to list drugs. And there is room for companies to apply for price adjustments after drugs have been listed.
* A 'joint medicines working group' will be set up-based on the same commercial principles which contribute to the high cost of medicines in the US. There include the 'need to recognize the value' of 'innovative pharmaceutical products' through strict intellectual property rights protection. Affordable access to medicines is not included.
* Australia has agreed to extend patent laws in some circumstances which will delay access to cheaper generics. Other changes make it easier for drug companies to raise legal objections and delay the production of generics. In the US, drug companies have used such legal tactics aggressively.
All these changes are subject to the 'disputes process' of the agreement. This means the US can complain to a tribunal of trade law experts if it believes that Australian laws or policies are not consistent with the agreement. The tribunal can order changes to the law backed up by trade sanctions.
Over 90 community organizations linked through the Australian Fair Trade and Investment Network (AFTINET) campaigned against the FTA, bolstering criticism of the deal by opposition parties. The Labor Party failed to keep its pledge to block the implementing legislation. But the Government did amend it to make sure US drug companies could not delay the availability of cheaper generic drugs.
In response, Washington and the companies demanded that the amendment be changed. The drug companies want the right to use the courts to delay access to cheaper medicines as they have in the US. They also attacked an election promise by the Government to lower prices paid to patent holders when cheaper generic drugs become available.
The newly re-elected government of John Howard is embarrassed by the US demands and is claiming the objections can be solved by negotiations-without changing the amendment.
At the time of writing, the two governments were still negotiating, having missed the 30 October deadline for signing off on the legislation.
Whatever the outcome, the deal confirms that the US model of free trade agreements limits the democratic right of peoples and governments to determine their own health and other social policies.
1 Trading in our Health System? The Australia Institute, Canberra, 2003, www.tai.org.au
Patricia Ranald is Principal Policy Officer with the Public Interest Advocacy Centre and Convenor of the Australian Fair Trade and Investment Network (AFTINET) www.aftinet.org.au
[ILLUSTRATION OMITTED]
COPYRIGHT 2004 New Internationalist Magazine
COPYRIGHT 2004 Gale Group