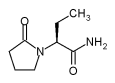
Brussels, 6 December 2005: According to new data, presented at a UCB
sponsored Scientific Exhibit at the 59th Annual Meeting of the
American Epilepsy Society in Washington, DC, U.S., approximately
one in four patients with poorly controlled idiopathic generalised
epilepsy (IGE) having primary generalised tonic-clonic (PGTC)
seizures became free from all types of seizures when Keppra®
(levetiracetam) was administered as an add-on treatment during a 20
week evaluation period. In comparison, only one in twelve of those
who took a placebo in addition to their usual therapy became seizure
free.
Study investigator, Dr Robert Leroy, from the Neurological Clinic of
Texas, commented, "These are major seizures which disrupt the
working, school and social lives of those who have them. The results
show that Keppra® significantly reduced PGTC seizures. The
tolerability profile is comparable to that seen in studies of Keppra®
in patients with epilepsy and other types of seizures."
All of the 164 patients aged 4 to 65 years enrolled in the trial had
at least three PGTC seizures during the eight weeks prior to
treatment, despite taking one or two anti-epileptic drugs (AEDs).
After a four-week prospective baseline period, the patients were
randomised to either Keppra®, up-titrated to 3000 mg/day in adults or
a target dose of 60 mg/kg/day in children, or placebo, followed by a
20 week stable dose period.
Treatment with Keppra® reduced the weekly PGTC seizure frequency
significantly more than placebo (p=0.004). In the Keppra® group, 72.2
% of patients had at least a 50% reduction from combined baseline in
PGTC seizure frequency per week during the treatment period, compared
with 45.2 % in the placebo group (p=0.0005). During the 20-week
evaluation period 24.1 % of patients treated with Keppra® became free
from all types of seizures compared with only 8.3% of placebo-treated
patients (p=0.009).
A preliminary assessment of safety data showed similar findings to
the established safety profile of Keppra®.
Notes to Editors
1. In a press release on October 26, 2005, UCB announced top-line
positive clinical trial results on this study evaluating Keppra®
(levetiracetam) as adjunctive treatment in adult and paediatric
patients (4-65 years of age) suffering from IGE with PGTC seizures.
2. Idiopathic generalised epilepsies (IGE) are a range of generalised
epilepsies for which there is no obvious cause, other than an
inherited (genetic) predisposition. They are characterised by
generalised tonic-clonic, myoclonic and absence seizures. Myoclonic
seizures are short, jerky muscle spasms that can occur singly or
repetitively, on one or both sides of the body. In a previous study,
Keppra® was shown to be highly effective as an adjunctive therapy in
IGE with myoclonic seizures, with 58.3% of patients (age 12-65 years)
achieving a reduction in myoclonic seizure days of at least 50%
compared with 23.3% in the placebo group (p=0.0002) [1].
UCB recently filed a variation application with the European
Medicines Agency (EMEA) and a supplemental new drug application
(sNDA) with the U.S. Food and Drug Administration (FDA) for the use
of Keppra® as adjunctive therapy in the treatment of myoclonic
seizures in patients, 12 years of age and older with juvenile
myoclonic epilepsy.
References
[1] Verdru, P., Wajgt, A., Schiemann Delgado J., Noachtar, S.
Efficacy and safety of levetiracetam 3000 mg/d as adjunctive
treatment in adolescents and adults suffering from idiopathic
generalised epilepsy with myoclonic seizures. Presented at the 26th
International Epilepsy Congress, Paris, August 2005
About Keppra®
In the U.S. and Europe, Keppra® (levetiracetam) is indicated as
adjunctive therapy in the treatment of partial onset seizures in
adults and children 4 years of age and older with epilepsy. In
adults, the use of Keppra® is associated with the occurrence of
central nervous system adverse events including somnolence and
fatigue, coordination difficulties, and behavioral abnormalities, as
well as hematological abnormalities. In paediatric patients 4 to 16
years of age, Keppra® is associated with somnolence, fatigue and
behavioural abnormalities, as well as hematological abnormalities. In
adults, the most common adverse events associated with Keppra® in
combination with other AEDs are somnolence, asthenia, infection, and
dizziness. Of these, most appeared to occur predominantly during the
first 4 weeks of treatment. In paediatric patients the most common
adverse events associated with Keppra® in combination with other AEDs
are somnolence, accidental injury, hostility, nervousness, and
asthenia.
For full U.S. prescribing information for Keppra® please visit
www.keppra.com
About UCB
UCB (www.ucb-group.com) is a global biopharmaceutical leader with
headquarters in Brussels, Belgium, specialising in the fields of
central nervous system disorders, inflammatory diseases, and
oncology. UCB key products are Keppra® (antiepileptic), Xyzal® and
Zyrtec® (antiallergics), Nootropil® (cerebral function regulator),
Tussionex® (antitussive) and Metadate(TM) / Equasym XL(TM) (attention
deficit/hyperactivity disorder). UCB employs over 8,500 people
operating in over 40 countries. UCB is listed on Euronext Brussels
(UCB / UCBBt.BR / UCB BB).
Copyright © Hugin ASA 2005. All rights reserved.