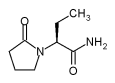
UCB Pharma, Inc. today announced that it filed a
supplemental new drug application (sNDA) with the U.S. Food and Drug
Administration (FDA) for the use of Keppra® (levetiracetam) as adjunctive
therapy in the treatment of myoclonic seizures in adults and adolescents 12
years of age and older with juvenile myoclonic epilepsy (JME). Keppra®
is currently approved for adjunctive therapy in the treatment of partial
onset seizures in adults and children 4 years of age and older with
epilepsy.
JME is a common type of epilepsy that requires life-long treatment with
anti-epileptic drugs (AEDs). While seizures associated with JME can
usually be controlled with medication, a large number of patients with JME
either don't respond to or cannot tolerate standard first-line treatments.
In addition, many JME patients are treated with inappropriate AEDs, which
are either ineffective against myoclonic seizures or associated with
unacceptable side effects.
This application for a new indication for Keppra® (levetiracetam) is
based on the first double-blind, randomized, placebo-controlled study
evaluating the efficacy and safety of an anti-epileptic drug in 120
patients (Keppra® N=60, placebo N=60) with idiopathic generalized
epilepsy (IGE) who experience myoclonic seizures.
"A surprising number of patients (up to 45%) do not respond to current
first line or newer therapies. This pivotal study shows that Keppra® may
be an effective treatment option for these patients that are difficult to
treat," said study investigator Gregory Krauss, Associate Professor,
Neurology, Johns Hopkins Medical School.
The study showed that Keppra® 3000 mg/day for 12 weeks reduced the rate
of myoclonic seizure days by at least half in 58% of treated patients
compared to 23.3% of placebo. A myoclonic seizure day was defined as any
day in which a patient experienced one or more myoclonic seizures.
Keppra® was generally well tolerated. Keppra® use was associated with
somnolence and behavioral abnormalities. The most common adverse events
not seen at an equivalent frequency among placebo patients were somnolence,
neck pain and pharyngitis.
According to Peter Verdru, M.D., Vice President, Clinical Research, Head of
Neurology/Psychiatry Clinical Development, UCB, "The new application
represents another milestone in the development of Keppra® as a major
advance in the management of epilepsy. No other anti-epileptic drug has
undergone such rigorous testing to determine its safety and efficacy in
patients suffering from idiopathic generalized epilepsy with myoclonic
seizures."
About Myoclonic Seizures and Juvenile Myoclonic Epilepsy
Myoclonic seizures are short, jerky muscle spasms that can occur once or
repetitively, on one or both sides of the body.
They occur in a variety of epilepsy syndromes that have different
characteristics. Myoclonic seizures are the hallmark symptom of a juvenile
myoclonic epilepsy (JME) diagnosis. JME is classified as a type of
idiopathic generalized epilepsy (IGE), in which seizures result from
excessive electrical activity in the whole brain. JME is a common type of
epilepsy that usually starts between the ages of 12 and 18, often around
puberty, and accounts for 10% of all cases of epilepsy. JME requires life-
long treatment with anti-epileptic drugs. Therefore, the goal of treatment
is to achieve long-term seizure freedom, without side effects of
medication. Myoclonic seizures are often overlooked, which may lead to
misdiagnosis and inappropriate treatment; this is thought to explain why
many patients with IGEs, such as JME, fail to respond to treatment.
About Keppra® (levetiracetam)
In the U.S., Keppra® is currently approved for adjunctive therapy in the
treatment of partial onset seizures in adults and children 4 years of age
and older with epilepsy. Keppra® is available in 250, 500 and 750 mg
tablets and a grape-flavored (100 mg/mL) oral solution for patients who
prefer a solution or have difficulty swallowing tablets. Keppra® dosing
must be individualized according to renal function status. Since its
launch, Keppra® has had more than 600,000 unique patient starts in the
United States.
In adults, Keppra® use is associated with the occurrence of central
nervous system adverse events, including somnolence and fatigue,
coordination difficulties, and behavioral abnormalities as well as
hematological abnormalities. In pediatric patients 4 to 16 years of age,
Keppra® is associated with somnolence, fatigue, and behavioral
abnormalities, as well as hematological abnormalities. In adults, the most
common adverse events associated with Keppra® in combination with other
AEDs were somnolence, asthenia, infection, and dizziness. Of these, most
appeared to occur predominantly during the first 4 weeks of treatment. In
pediatric patients 4 to 16 years of age, the most common adverse events
associated with Keppra® in combination with other AEDs were somnolence,
accidental injury, hostility, nervousness, and asthenia.
Keppra® (levetiracetam) was approved in 1999 as adjunctive therapy for
adults with partial onset seizures and is the most prescribed second-
generation AED used in epilepsy. For full prescribing information, please
visit www.keppra.com.
As The Epilepsy Company, UCB has many programs for patients with epilepsy
and their caregivers, including a dedicated web site geared toward epilepsy
education. By logging onto ucbepilepsy.com, patients can obtain a variety
of resources including a downloadable seizure diary and application forms
for the newly established UCB scholarship program, which is unique as it
provides financial assistance for epilepsy patients, their families and
caregivers. UCB is also a proud sponsor of the Epilepsy Foundation's
H.O.P.E. Mentoring Program(TM) (Helping Other People with Epilepsy) that
trains people with epilepsy to be "patient educators" throughout the
epilepsy and neurology communities. H.O.P.E. mentors also conduct
educational sessions for local social and civic groups in the community.
About UCB
UCB S.A. (www.ucb-group.com) is a global biopharmaceutical leader dedicated
to the research, development, and commercialization of innovative products
in the fields of central nervous system disorders, allergy and respiratory
diseases, immune and inflammatory disorders and oncology. UCB employs over
8,500 people operating in over 40 countries, and achieved revenues of EUR
2.1 billion (including net turnover, royalties, and fees) in 2004. UCB is
listed on the Euronext Brussels with a market capitalization of
approximately EUR 5.5 billion. Worldwide headquarters are located in
Brussels, Belgium.
UCB Pharma, Inc. is the North American subsidiary of UCB S.A., with U.S.
headquarters located in Smyrna, Georgia. UCB key products in the U.S. are
Keppra® (levetiracetam), Zyrtec®† (cetirizine HCl), Tussionex® CIII
(hydrocodone polistirex/chlorpheniramine polistirex), and Metadate CD(TM)
CII (methylphenidate HCl, USP).
† Zyrtec is licensed to and co-promoted with Pfizer, Inc. in the United
States.
*Keppra® (levetiracetam) is a registered trademark of the UCB S.A.
Contact:
Lisa Garman
UCB Pharma, Inc.
770-970-8569
Email Contact
Judi Kennedy
Chandler Chicco Agency
212-229-8439
Email Contact