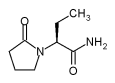
UCB Pharma, Inc. announced today that the U.S.
Food and Drug Administration (FDA) has approved the company's anti-epilepsy
drug Keppra® (levetiracetam) as add-on therapy in the treatment of
partial-onset seizures in children four years of age and older with
epilepsy. The FDA approved this new pediatric indication for Keppra®
under a six-month priority review.
"More than 25% of children with epilepsy experience treatment resistant
seizures or intolerable side effects from medication," said Tracy Glauser,
M.D., director of the Comprehensive Epilepsy Program, Cincinnati Children's
Hospital, and principal investigator of the well-controlled study reviewed
for the pediatric indication. Dr. Glauser added that, "Keppra® was
effective and well tolerated by the children in the study, many of whom had
failed on multiple anti-epileptic drugs (AEDs) prior to trying Keppra®."
"This approval provides another needed treatment option for children who
suffer from epilepsy. We applaud the FDA for making this therapeutic
option available," said Eric Hargis, President of the Epilepsy Foundation.
Clinical Trial Results
The approval of Keppra® (levetiracetam) for children was based on
findings from one multi-center, randomized, double-blind,
placebo-controlled pivotal study conducted at 60 sites in North America, in
198 children 4 to 16 years of age with partial onset seizures with or
without secondary generalization uncontrolled by standard AEDs.(1) Study
participants were taking one or two other AEDs at entry. The study
consisted of an 8-week baseline period and a 4-week titration period,
followed by a 10-week evaluation period.
When measuring efficacy, those taking Keppra® had a significantly larger
reduction (26.8%) in weekly seizure frequency over placebo, on average.
Additionally, responder rates (the portion of patients achieving a 50% or
greater reduction in seizures) for patients taking Keppra® were 44.6%
versus 19.6% for placebo (both with a p=0.0002 compared to placebo).
"We are very pleased the FDA approved Keppra® for children, and look
forward to making this therapy available for many of the 300,000 children
in the U.S. with epilepsy," said Peter Verdru, M.D., Vice President
Clinical Research and head of Neurology, Psychiatry and Clinical
Development, UCB Pharma, Inc.
In pediatric patients, 4 to 16 years of age, the most common adverse events
associated with Keppra® in combination with other AEDs were somnolence,
accidental injury, hostility, nervousness and asthenia. Keppra® is
associated with somnolence, fatigue, and behavioral abnormalities as well
as hematological abnormalities.
About Keppra®
In the U.S., Keppra® is approved for adjunctive therapy in the treatment
of partial onset seizures in adults and children 4 years of age and older
with epilepsy. Keppra® is available in 250, 500 and 750 mg tablets and a
grape-flavored (100 mg/mL) oral solution for patients who prefer a solution
or have difficulty swallowing tablets. Keppra® dosing must be
individualized according to renal function status. Since its launch,
Keppra® has had more than 600,000 unique patient starts in the United
States.(2)
In adults, Keppra® (levetiracetam) use is associated with the occurrence
of central nervous system adverse events, including somnolence and fatigue,
coordination difficulties, and behavioral abnormalities as well as
hematological abnormalities. In well-controlled adult clinical studies,
the most frequently reported adverse events associated with the use of
Keppra® in combination with other AEDs, not seen at an equivalent
frequency among placebo-treated patients, were somnolence, asthenia,
infection and dizziness.
Keppra® was approved in 1999 as adjunctive therapy for adults with
partial onset seizures and is the most prescribed second-generation AED
used in epilepsy.(3) For full prescribing information please visit
www.keppra.com.
About UCB
UCB (www.ucb-group.com) is a global biopharmaceutical leader dedicated to
the research, development, and commercialization of innovative products in
the fields of central nervous system disorders, allergy and respiratory
diseases, immune and inflammatory disorders and oncology. UCB employs over
8,500 people operating in over 40 countries, and achieved revenues of EUR
2.1 billion (including net turnover, royalties, and fees) in 2004. UCB is
listed on the Euronext Brussels with a market capitalization of
approximately EUR 5.5 billion. Worldwide headquarters are located in
Brussels, Belgium.
UCB Pharma, Inc. is the North American subsidiary of UCB S.A., with U.S.
headquarters located in Smyrna, Georgia. UCB's key products in the U.S. are
Keppra® (levetiracetam), Zyrtec®† (cetirizine HCl), Tussionex® CIII
(hydrocodone polistirex/chlorpheniramine polistirex), and Metadate® CD
CII (methylphenidate HCl, USP).
K354-0605
Contact:
Lisa Garman
UCB Pharma, Inc.
770-970-8569
Email Contact
Judi Kennedy
Chandler Chicco Agency
212-229-8439
Email Contact