METHOD OF PREPARATION
Note: This preparation should he done in a laminar airflow hood in a cleanroom or via isolation barrier technology by a validated aseptic compounding pharmacist using strict aseptic technique.
1. Calculate the required quantity of each ingredient for the total amount to be prepared.
2. Accurately weigh and/or measure each ingredient.
3. Aseptically, obtain the required quantity of ketamine and fentanyl and mix well in a suitable sterile container.
4. Aseptically, add sufficient 0.9% sodium chloride injection to volume and mix well.
5. Package and label.
PACKAGING
Package in tight, light-resistant containers.1
LABELING
Keep out of reach of children. Use only as directed.
STABILITY
If no sterility testing is done, a beyond-use date of 24 hours at room temperature, 3 days at refrigerated temperature or 45 days at
USE
Ketamine and fentanyl in combination are used in the treatment of moderate-to-severe pain.
QUALITY CONTROL
Quality-control assessment can include weight/volume, physical observation, pH, specific gravity, osmolality, assay, color, clarity, particulate matter, sterility and pyrogenicity.3,4
DISCUSSION
Ketamine hydrochloride injection is available in concentrations equivalent to 10, 50 or 100 mg/mL of ketamine base. The pH of the injection is from 3.5 to 5.5. The injection also contains 0.1 mg/mL of benzethonium chloride, and the 10 mg/mL concentration is rendered isotonic with sodium chloride.
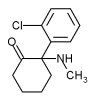
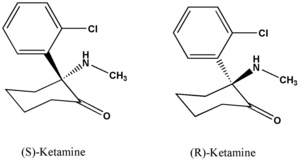
Ketamine hydrochloride (C^sub 13^H^sub 16^ClNO.HCl, MW 274.2) is used as an anesthetic and analgesic. It occurs as a white crystalline powder with a slight characteristic odor. Approximately 1.15 mg is equivalent to 1 mg of ketamine base. It is soluble 1 g in 4 mL of water, 14 mL of alcohol and 60 mL of absolute alcohol. Ketamine hydrochloride injection is a sterile solution of ketamine hydrochloride in water for injection. It contains an amount of ketamine hydrochloride equivalent to not less than 95.0% and not more than 105.0% of the labeled amount of ketamine. The injection has a pH of 3.5-5.5, and it contains not more than 0.4 USP endotoxin units per mg of ketamine hydrochloride. It should be stored at controlled room temperature and protected from light.1,5
Fentanyl citrate (C^sub 22^H^sub 28^N^sub 2^O.C^sub 6^H^sub 8^O^sub 7^, MW 528.6) occurs as white granules or as a white crystalline powder. Fentanyl citrate injection contains fentanyl, as the citrate 50 µg per mL with hydrochloric acid and/or sodium hydroxide to adjust pH to the range of 4 to 7.5. Fentanyl citrate 157 µg is approximately equal to 100 µg of fentanyl. It is soluble 1 g in 40 mL of water and slightly soluble in alcohol. The pH of the fentanyl injection is in the range of pH 4.0 to 7.5. Fentanyl citrate is a synthetic opioid analgesic used as a sedative, analgesic, preoperative medication and an adjunct to general or regional anesthesia and in the management of chronic pain.6
0.9% Sodium chloride injection contains not less than 95.0% and not more than 105.0% of the labeled amount of sodium chloride in water for injection. It has a pH between 4.5 and 7.0 and contains no added antimicrobial agents. They can be sterilized by filtration or autoclaving. Sodium chloride will decrease the solubility of some organic compounds; methylparaben is not as soluble in sodium chloride solutions as it is in water. Sodium chloride is soluble in water to the extent of 1 g in 2.8 mL water, and is slightly soluble in alcohol (1 g in 250 mL of 95% ethanol).1
REFERENCES
1. US Pharmacopeial Convention, Inc. United States Pharmacopeia 27-National Formulary 22. Rockville, MD: US Pharmacopeial Convention, Inc.; 2004: 1054-1056, 1700, 2345-2349, 2768.
2. Ambados F, Brealey J. Compatibility of ketamine hydrochloride and fentanyl citrate in polypropylene syringes. Am J Health Syst Pharm 2004; 61: 1438-1439.
3. Allen LV Jr. Standard operating procedure for particulate testing for sterile products. IJPC 1998; 2: 78.
4. Allen LV Jr. Standard operating procedure: Quality assessment for injectable solutions. IJPC 1999; 3: 406-407.
5. Sweetman SC, ed. MARTINDALE: The Complete Drug Reference. 33rd ed. London: Pharmaceutical Press; 2002: 1262-1263.
6. Reynolds JEF, ed. MARTINDALE: The Extra Pharmacopoeia. 30th ed. London: Pharmaceutical Press; 1993: 1076-1077.
Copyright International Journal of Pharmaceutical Compounding Nov/Dec 2004
Provided by ProQuest Information and Learning Company. All rights Reserved