METHOD OF PREPARATION
Note: This preparation should be done in a laminar airflow hood in a cleanroom or via isolation barrier technology by a validated aseptic compounding pharmacist using strict aseptic technique.
1. Calculate the required quantity of each ingredient for the total amount to be prepared.
2. Accurately weigh and/or measure each ingredient.
3. Heat about 90 mL of purified water to about 90°C, add the methylparaben and propylparaben and stir until dissolved.
4. Cool to room temperature and add the ketamine hydrochloride and sodium chloride and mix until dissolved.
5. Add sufficient purified water to volume and mix well.
6. Filter through an appropriate sterile 0.2-µm filter into sterile containers.
7. Package and label.
PACKAGING
Package in tight, light-resistant containers.1
LABELING
Keep out of reach of children. Use only as directed. For nasal use only.
STABILITY
A beyond-use date of 48 hours at room temperature, 14 days at refrigerated temperature or 45 days at
USE
Ketamine nasal spray is used in the treatment of pain. The dose can be measured by calibrating the spray bottle that is used.
QUALITY CONTROL
Quality-control assessment can include weight/volume, physical observation, pH, specific gravity, osmolality, assay, clarity and sterility.2,3
DISCUSSION
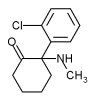
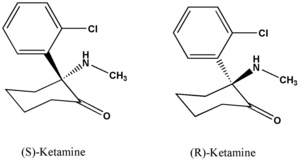
Ketamine hydrochloride (C^sub 13^H^sub 16^ClNO.HCl, MW 274.2) is used as an anesthetic and analgesic. It is usually administered parenterally but is also used as a nasal spray in patients where the patenterai route is not convenient. Ketamine hydrochloride occurs as a white crystalline powder with a slight characteristic odor. Approximately 1.15 mg is equivalent to 1 mg of ketamine base. It is soluble 1 g in 4 mL of water, 14 mL of alcohol and in 60 mL of absolute alcohol. Ketamine hydrochloride injection is a sterile solution of ketamine hydrochloride in water for injection. It contains an amount of ketamine hydrochloride equivalent to not less than 95.0% and not more than 105.0% of the labeled amount of ketamine. The injection has a pH of 3.5-5.5 and it contains not more than 0.4 USP endotoxin units per mg of ketamine hydroehloride. It should be stored at controlled room temperature and protected from light.1,4
Sodium chloride (NaCl, MW 58.44) is available as a white crystalline powder or as colorless crystals. In parenteral, ophthalmic and nasal preparations, it is used to prepare isotonic solutions. It is soluble in water (1 g in 2.8 mL), glycerin (1 g in 10 mL) and 95% ethanol (1 g in 250 mL). A 0.9% w/v aqueous solution is iso-osmotic with serum and its solutions are stable.1
Methylparaben (C^sub 8^H^sub 8^O^sub 3^, MW 152.15, methyl hydroxybenzoate, methyl parahydroxybenzoate) is available as colorless crystals or as a white, crystalline powder that is odorless or almost odorless, and has a slight burning taste. One gram is soluble in 400 mL of water, 3 mL of 95% ethanol, 60 mL glycerin, 200 mL peanut oil and 5 mL propylene glycol.6
Propylparaben (C^sub 10^H^sub 12^O^sub 3^, MW 18.20, propyl hydroxybenzoate, propyl parahydroxybenzoate) is available as a white, crystalline, odorless and tasteless powder. One gram is soluble in 2500 mL of water, 1.1 mL ethanol, 250 mL glycerin, 70 mL peanut oil and 3.9 mL propylene glycol.7
Purified water is water that is obtained by distillation, ion exchange, reverse osmosis or some other suitable process.8
REFERENCES
1. US Pharmacopeial Convention, Inc. United States Pharmacopeia 27-National Formulary 22. Rockville, MD: US Pharmacopeial Convention, Inc.; 2004: 1262-1263, 2345-2349.
2. Allen LV Jr. Standard operating procedure for particulate testing for sterile products. IJPC 1998; 2: 78.
3. Allen LV Jr. Standard operating procedure: Quality assessment for injectable solutions. IJPC 1999; 3: 406-407.
4. Sweetman SC, ed. MARTINDALE: The Complete Drug Reference. 33rd ed. London: Pharmaceutical Press; 2002: 1262-1263.
5. Cable CG. Sodium chloride. In: Kibbe AH, ed. Handbook of Pharmaceutical Excipients. 3rd ed. Washington, DC: American Pharmaceutical Association; 2000: 478-481.
6. Reiger MM. Methylparaben. In: Rowe RC, Sheskey PJ, Weller PJ, eds. Handbook of Pharmaceutical Excipients. 4th ed. Washington, DC: American Pharmaceutical Association; 2003: 390-394.
7. Rieger MM. Propylparaben. In: Kibbe AH, ed. Handbook of Pharmaceutical Excipients. 3rd ed. Washington, DC: American Pharmaceutical Association; 2000: 450-453.
8. Ellison A, Nash RA, Wilkin MJ. Water. In: Rowe RC, Sheskey PJ, Weller PJ, eds. Handbook of Pharmaceutical Excipients. 4th ed. Washington, DC: American Pharmaceutical Association; 2003: 672-676.
Copyright International Journal of Pharmaceutical Compounding Nov/Dec 2004
Provided by ProQuest Information and Learning Company. All rights Reserved