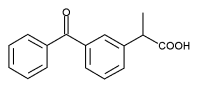
Acute renal failure induced by topical ketoprofen
T Krummel, Y Dimitrov, B Moulin, T Hannedouche, Department of Nephrology, Hopitaux Universitaires, Strasbourg, France
Acute renal failure is a known complication with oral nonsteroidal anti-inflammatory drugs.[1 2] In contrast, topical application of such drugs is usually considered safe because of marginal percutaneous absorption (5-8%).[3] We report a case of acute renal failure after treatment with topical ketoprofen.
A 62 year old Turkish woman with chronic renal failure from polycystic kidney disease had had stable renal function for several months, with a creatinine concentration of 360 [micro]mol/l, and stable treatment, (rilmenidine, furosemide, atenolol, amlodipine, enalapril, aspirin, sodium bicarbonate, simvastatin, dietary salt restriction). She was admitted for inflammatory arthritis of one ankle and was treated with colchicine (1 mg/day), without digestive intolerance, and topical ketoprofen twice daily for five days. Her serum creatinine and urea concentrations rose from 390 [micro]mol/l and 25 mmol/l to 673 [micro]mol/l and 38 mmol/l respectively by the fifth day of treatment. Diuresis was unchanged, but urinary sodium and fractional sodium excretion fell, suggesting functional acute renal failure. No urinary or blood eosinophilia were found. Treatment with ketoprofen, enalapril, and furosemide were immediately stopped, and her renal function returned to its initial level after eight days. Her blood concentration of free unbound ketoprofen was 4.17 mg/l six days after stopping ketoprofen (normal range for oral ketoprofen 5-10 mg/l).
Systemic non-steroidal anti-inflammatory drugs can induce renal failure in two ways. Acute interstitial nephritis, with or without nephrotic syndrome, is relatively rare and is presumably due to a dose independent allergic mechanism.[1] Non-selective inhibition of cyclo-oxygenases 1 and 2, causes a functional reversible acute renal failure. Cyclo-oxygenase 2 is selectively induced by proinflammatory cytokines at inflammation sites, but cyclo-oxygenase 1 is constitutionally expressed in many tissues, including the kidney, especially in situations in which renal perfusion is compromised. In these situations oral non-steroidal anti-inflammatory drugs are contraindicated.
In this case, chronic renal failure, treatment with angiotensin converting enzyme inhibitors and diuretics, and age were all high risk factors for acute renal failure with non-steroidal anti-inflammatory drugs.[1 2] The renal was probably due to ketoprofen treatment, given the rise in serum creatinine concentration and high blood concentration of ketoprofen detected. This case illustrates that transdermal absorption of ketoprofen can cause high blood concentrations. Renal failure is a predisposing condition as it can reduce clearance of ketoprofen.[4] Acute renal failure after treatment with topical non-steroidal anti-inflammatory drugs has been reported previously: two cases after application of piroxicam and ibuprofen (probably allergic reactions), and another after treatment with 3% benzydamine.[2 5] None reported blood concentrations of the drugs.
This case shows that topical treatment with ketoprofen can result in blood concentrations and systemic effects comparable to those from oral treatment. Topical non-steroidal anti-inflammatory drugs should be used with the same precautions as other forms of the drugs in high risk patients, especially those with reduced drug metabolism as in renal failure.
[1] Sandier D, Burr R, Weinberg C. Nonsteroidal anti-inflammatory drugs and the risk for chronic renal disease. Ann Intern Med 1991; 115(3): 165-72.
[2] O'Callaghan CA, Andrews PA, Ogg C. Renal disease and use of topical non-steroidal anti-inflammatory drugs. BMJ 1994;308:110-1.
[3] Shah A, Wei G, Lanman R, Bhargava V, Weir S. Percutaneous absorption of ketoprofen from different anatomical sites in man. Pharm Res 1996;13(1):168-72.
[4] Skeith. KJ, Dasgupta M, Lange R, Jamali E The influence of renal function on the pharmacokinetics of unchanged and acyl-glucuroconjugated ketoprofen enantiomers after 50 and 100 mg racemic ketoprofen. Br J Clin Pharmacol 1996; 42:163-9.
[5] Fernando A, Thomas S, Temple R, Lee H. Renal failure after topical use of NSAIDs. BMJ 1994;308:533.
COPYRIGHT 2000 British Medical Association
COPYRIGHT 2000 Gale Group