METHOD OF PREPARATION
Note: It is generally recommended to prepare sufficient material for one or two additional suppositories due to loss during preparation. It may be necessary to calibrate the desired mold to be used prior to preparing this prescription.
1. Calculate the required quantity of each ingredient for the total amount to be prepared.
2. Accurately weigh and/or measure each ingredient.
3. Using a source of low heat, melt the required quantity of base for the number of suppositories to be prepared.
4. Slowly sprinkle the ketoprofen and the silica gel powders into the melt with stirring until a uniform mixture is obtained.
5. Cool slightly, then pour into the mold in a continuous motion, if appropriate for the molds being used.
6. Cool, trim if necessary, package and label.
PACKAGING
Package in tight, light-resistant containers.1
LABELING
Keep out of reach of children. Use only as directed. For rectal use. Store in a refrigerator.
STABILITY
A beyond-use date of up to 6 months is appropriate for this preparation.1
USE
Ketoprofen rectal suppositories are used in the treatment of mild to moderate pain when a nonsteroidal anti-inflammatory agent (NSAID) is desired.
QUALITY CONTROL
Quality-control assessment can include weight, specific gravity, active drug assay, color, texture of surface, appearance, feel, melting test, physical observation and physical stability.2
DISCUSSION
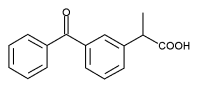
Ketoprofen (C^sub 16^H^sub 14^O^sub 3^, MW 254.28) occurs as a white or almost-white, odorless or almost-odorless, crystalline powder. It is practically insoluble in water but freely soluble in alcohol and ether. It has a melting range between 92.0° and 97.0°C. Ketoprofen has analgesic, anti-inflammatory and antipyretic properties and is an inhibitor of cyclo-oxygenase. It should be preserved in tight containers.3
Silica gel, a form of silicon dioxide, occurs as a line white, hygroscopic, odorless, amorphous powder with a usual particle size range between 2 µm and 10 µm. It is insoluble in water and alcohol and other organic solvents. Tt is used as a desiccant and viscosity-increasing agent.1,4
Witepsol H15 is part of a family of about twelve Witepsol bases that are nearly white and almost odorless. Witepsol 1115 has a melting range and release characteristics similar to that of cocoa butter. It solidifies rapidly in the mold, and lubrication is not necessary because the suppositories contract well and are easily removed.5
Fatty-acid base is a preblended suppository base that is used when a fatty-acid base is preferred, occurring as an opaque white solid. It contains triglycerides derived from palm, palm kernel and coconut oils with self-emulsifying glyceryl monostearate and polyoxyl stearate as emulsifying and suspending agents. It is stable, has a bland taste and odor and a controlled melting range. It is widely used as a cocoa butter replacement for suppositories, lipsticks and lip balms. It has a melting point between 35° and 37°C and a specific gravity of 0.890.6
Cocoa butter (Theobroma oil) is a yellowish or white-colored brittle solid with a slight odor of cocoa. It is derived from natural sources and is composed primarily of triglycerides of saturated and unsaturated fatty acids. It melts between 31 ° and 34°C, is freely soluble in chloroform and ether and slightly soluble in 95% ethanol. Heating cocoa butter to a temperature greater than 36°C results in a lowering of the solidification point because of its polymorphic nature and the formation of a metastable form. It should be stored at temperatures less than 2S°C. It is used as a suppository base and is also a major ingredient in chocolate.7
REFERENCES
1. US Pharmacopeial Convention, Inc. United States Pharmacopeia 27-National Formulary 22. Rockville, MD: US Pharmacopeial Convention, Inc; 2004: 2345-2349, 2927-2928.
2. Allen Jr LV. Standard operating procedure for performing physical quality assessment of suppositories, troches, lollipops and sticks. IJPC 1999; 3(1): 56-57.
3. Reynolds JEF, ed. MARTINDALE: The Extra Pharmacopoeia. 30th ed. London: The Pharmaceutical Press; 1993; MART 21-22.
4. Morefield E. Colloidal silicon dioxide. In: Kibbe AH, ed. Handbook of Pharmaceutical Excipients. 3rd ed. Washington, DC: American Pharmaceutical Association; 2000: 143-145.
5. Allen Jr LV. Compounding Suppositories, Part II. Secundum Ariern; 3 (3): 3.
6. Fatty Acid Base [product information]. Minneapolis, MN: Paddock Laboratories, Inc.
7. Reilly Jr WJ. Pharmaceutical necessities. In: Gennaro AR, ed. Remington: The Science and Practice of Pharmacy. 19th ed. Easton, PA: Mack Publishing Company; 1995: 1409.
Copyright International Journal of Pharmaceutical Compounding May/Jun 2004
Provided by ProQuest Information and Learning Company. All rights Reserved