ABSTRACT
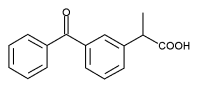
Ketoprofen (3-benzoyl-[alpha]-methylbenzeneacetic acid, KP) is a widely used nonsteroidal anti-inflammatory drug (NSAID) that causes both phototoxicity and photoallergy. Here, we investigated the formation of hemoglobin radicals, in both purified hemoglobin and red blood cells (RBC), induced by ultraviolet A (UVA)-KP by using "immuno-spin trapping," a novel approach that combines the specificity of spin trapping with the sensitivity of antigen-antibody interactions. The methemoglobin (metHb) radicals react covalently with 5,5-dimethyl-1-pyrroline N-oxide (DMPO) to form nitroxyl radical adducts that are oxidized to the corresponding nitrone adducts, which in turn are specifically recognized by antiserum against DMPO nitrone. We found that the formation of nitrone adducts in metHb depended on the UVA dose, the KP concentration and the presence of DMPO, as determined by enzyme-linked immunosorbent assay and Western blotting. Adduct formation decreased when irradiation was carried out in the presence of catalase or nitrogen, suggesting that H^sub 2^O^sub 2^ plays a key role in KP-UVA-induced metHb radical formation. KP in the dark did not generate metHb radical-derived nitrone adducts, whereas UVA alone resulted in the formation of metHb radical-derived nitrone adducts that increased with UVA dose from 4 to 10 J/cm^sup 2^. However, KP (25 and 200 [mu]M) plus UVA (4 and 10 J/cm^sup 2^) resulted in a significant increase in the formation of metHb radical-derived nitrone adducts as compared with UVA or KP alone, indicating that KP photosensitized the production of the metHb radicals in the presence of UVA. In contrast, no metHb radical-derived nitrone adduct was detected in the absence of DMPO, even though KP and UVA were present. We also detected the hemoglobin radical formation in RBC as well as in hemolysates. The endogenous antioxidants and exogenous reduced glutathione inhibited the protein radical formation. These studies have shown that the immuno-spin-trapping technique can be used to detect radical damage in proteins as a result of photosensitizing reactions. The successful detection of protein radical formation caused by KP photosensitization could help further understand the photoallergic effect of this NSAID.
INTRODUCTION
The nonsteroidal anti-inflammatory drug ketoprofen (KP) (Scheme 1) is an arylpropionic acid derivative widely used for the treatment of rheumatic diseases (1). Recent studies have shown that KP causes photoallergic dermatitis and phototoxic effects (2-4). Photoallergic effects, in particular, are presumed to result from damage to proteins, which then act as allergens.
When ultraviolet (UV) or visible light damages proteins, they often form free radical intermediates that can, in principle, be detected using electron spin resonance (ESR) spin trapping. However, recently, an antiserum directed against the nitrone spin trap 5,5-dimethyl-1-pyrroline N-oxide (DMPO) was developed and validated (5). This new approach, immuno-spin trapping, which combines the specificity of spin trapping with the sensitivity of antigen-antibody interactions, has several advantages over ESR spin trapping (for discussion see Detweiler et al. [5] and Ramirez et al. [6]). To test the ability of immuno-spin trapping to detect light-induced protein damage, we used a system containing KP, hemoglobin or erythrocytes and DMPO.
We chose KP as a photosensitizer because it is known to induce hemolysis of erythrocytes upon UVA irradiation. Furthermore, KP acts via a mechanism that does not photogenerate KP-derived radical intermediates that are trapped by DMPO (7). Thus, when KP is photolysed in the presence of protein and DMPO, the DMPO will not prevent KP or its photoproducts from generating protein-derived radicals (7,8).
As a test protein we focused on hemoglobin, which has several advantages. It is abundant in erythrocytes and is known to form free radicals when damaged by H^sub 2^O^sub 2^, as detected by ESR spectroscopy (9). Its free radical products are known to react with DMPO; DMPO has been shown to form radical adducts with sperm whale (10) and human metmyoglobins (metMb) (11,12). These adducts are known to be recognized by the anti-DMPO serum (5).
To date, there have been no reports on protein radical production induced by KP photosensitization. In the present work we report that photoirradiated KP reacts with hemoglobin in various forms to produce radicals that can be detected by the immuno-spin-trapping technique. We have also tested KP as a source of protein damage in intact cells.
MATERIALS AND METHODS
Materials. KP, human methemoglobin (metHb), oxyhemoglobin (oxyHb), horse skeletal muscle (metMb), diethylenentriamine pentaacetic acid (DTPA), superoxide dismutase (SOD), and reduced glutathione (GSH) were purchased from Sigma Chemical Co. (St. Louis, MO). Beef liver catalase was purchased from Roche Molecular Biochemicals (Indianapolis, IN). The spin-trap DMPO from Aldrich Chemical Co. (Chicago, IL) was vacuum distilled and stored at -20[degrees]C until use. All buffers were treated with Chelex 100(R) (Bio-Rad Laboratories, Hercules, CA) at 4[degrees]C for 24 h followed by addition of 100 [mu]M DTPA to avoid possible transition metal-catalyzed reactions.
Hemoglobin preparation. Purified metHb, metMb or oxyHb (oxyferrous form) was dissolved in 0.1 M phosphate buffer, pH 7.4, containing DTPA (100 [mu]M) and treated with Chelex 100(R). Before use the preparations of oxyHb and metHb were passed through a prepacked Sephadex G-25 column (PD-10; Amersham Pharmacia Biotech, Piscataway, NJ) equilibrated with 0.1 M phosphate buffer, and the contribution of each oxyHb and metHb form in the respective preparations was determined to be higher than 95% (13). In this study, the protein concentration in all hemoglobin solutions (as heme) was quantified by adding an excess of sodium dithionite and by using the absorbance of deoxyhemoglobin at 430 and 555 nm ([epsilon]430 = 133 mM^sup -1^ cm^sup -1^ and [epsilon]555 = 12.5 mM^sup -1^ cm^sup -1^, respectively) at pH 7.4.
Red blood cells. Outdated human erythrocytes were obtained from the American Red Cross, Durham, NC. Red blood cell (RBC) pellets were washed three times with physiological saline (NaCl, 0.85% wt/vol) and resuspended in 1% Chelex-treated 0.1 M phosphate buffer containing 100 [mu]M DTPA. The RBC were counted in a hemocytometer. An RBC suspension that contained 10 [mu]M hemoglobin was used for treatment. Before analysis, some samples were lysed by three freeze-thaw cycles. Other samples were centrifuged at 500 g for 5 min to separate the reaction supernatant (hemolysate) and intact RBC.
Preparation of hemolysate. To prepare hemolysate, a 1% RBC suspension was centrifuged at 3000 rpm for 5 min, and the cell pellet was resuspended in Chelex-100-treated 10 mM sodium phosphate buffer, pH 7.4, containing 100 [mu]M DTPA and incubated for 15 min on ice. After centrifugation to eliminate cell debris, hemoglobin was separated and filtered through a prepacked Sephadex G-25 column (PD-10; Amersham Pharmacia Biotech) equilibrated with 0.1 M phosphate buffer, pH 7.4.
UVA treatment. The reaction mixture with or without KP was irradiated with fluorescent lamps (Houvalite F20T12BL-HO PUVA; National Biological Corp., Twinsburg, OH) in an eight-well plate with the dish lid on. The UVA dose was monitored with a Goldilux UV meter equipped with a UVA detector (Oriel Instruments, Stratford, CT). Control samples were kept in the dark under the same conditions. UVA of 10 J/cm^sup 2^ equates to about 30 min in the midday sun during summer at latitude 48'N (14). After treatment, the samples were kept at -70[degrees]C until analysis.
Hemolysis determination. After treatment, supernatant was centrifuged to remove intact RBC. The protein released into the supernatant was therefore determined by bicinchoninic assay (Pierce, Rockford, IL). The total protein content in RBC was measured after complete lysis of RBC by sonication. Hemolysis was expressed as a percentage of the control cells without treatment with KP or UVA.
Enzyme-linked immunosorbent assay. Hemoglobin radical-derived nitrone adducts were determined using standard enzyme-linked immunosorbent assay (ELISA) in 96-well plates (Greiner Labortechnik, Frickenhausen, Germany) as described previously (5). Briefly, the adduct solution (1 [mu]g protein) in coating buffer was incubated for 90 min at 35[degrees]C.
After blocking, rabbit anti-DMPO serum (1:5000) (5) was added and incubated for 60 min at room temperature. After washing, the secondary antibody, anti-rabbit IgG-alkaline phosphatase (Pierce; 1:5000 in washing buffer), was added and incubated for 60 min at room temperature. The antigen-antibody complexes were developed by using a chemiluminescence system (CDP-Star; Roche Molecular Biochemicals), and the light emitted was measured in arbitrary units using Xfluor Software (Tecan US, Research Triangle Park, NC).
Western blotting analysis. After UVA irradiation, the proteins (1.2 [mu]g/lane) were separated on 4-12% Bis-Tris NuPAGE (Invitrogen, Carlsbad, CA) and then transferred to a nitrocellulose membrane. After blocking for 90 min, the membrane was incubated with the primary (rabbit anti-DMPO serum, 1:5000) and the secondary (anti-rabbit IgG conjugated with alkaline phosphatase) antibodies. The protein products were observed by enhanced chemiluminescence (Nitro Block II; Tropix, Bradford, MA; CDP-Star II).
Hydrogen peroxide assay. Hydrogen peroxide was assayed using the Amplex Red kit from Molecular Probes (Eugene, OR) according to the supplier's directions.
Spin trapping. EPR spectra were recorded using a Varian E-109 Century line spectrometer operating at 9.5 GHz with 100 kHz modulation. Unless otherwise indicated, the following instrumental settings were used: microwave power, 10 mW; modulation, 0.33 G; time constant, 0.25 s; scan time, 4 min. Samples were placed in a quartz flat cell and irradiated directly inside the microwave cavity of the spectrometer by using a 1 kW Xe arc lamp. Radiation from the lamp was passed through a filter (window glass) to remove wavelengths below 300 nm. Hyperfine couplings were obtained by accumulating, simulating and optimizing spectra on an IBM PC computer, using software described elsewhere (15).
Chemical modification of metHb by iodination of tyrosine residues. The reaction mixture contained 1 mL aliquots of metHb (200 [mu]M) in 50 [mu]M sodium phosphate buffer, pH 7.4, and NaI (final concentration of 40 mM). Iodination of tyrosine residues of metHb was initialized by the addition of two N-chloro-benzenesulfonamide immobilized beads (IODO-BEADS; Pierce Chemical Co.). The reaction was allowed to proceed with shaking at 23[degrees]C for 15 min. To stop the reaction, the solution was removed from the vessel and passed through a prepacked Sephadex G-25 column (PD-10; Amersham Pharmacia Biotech) to remove excess NaI. The iodinated metHb fraction was collected, and its concentration was quantified by adding excess of sodium dithionite and using the absorbance of deoxyhemoglobin at 430 nm ([epsilon]430 = 133 mM^sup -1^ cm^sup -1^).
Blocking of thiol groups in metHb. A suspension of 5,5'-dimiobis(2-nitrobenzoic acid) (0.2 M) was prepared in 50 mM sodium phosphate buffer and mixed with the metHb at a molar ratio of 100:1. The reaction was allowed to proceed for 10 min at room temperature. The reaction mixture was then dialyzed (Slide-A-Lyzer; Pierce Chemical Co.) overnight against 50 mM sodium phosphate buffer (pH 7.4) with one change of buffer.
Statistics. Data are presented as mean + or - standard error of the mean of three to six experiments. The Student's t-test was used for comparisons between experimental groups (n = 3 to 6). A value of P
RESULTS
KP-UVA-induced production of metHb radical nitrone adducts detected by ELISA
KP photosensitization resulted in the production of metHb radical nitrone adducts, which were detected by ELISA after reacting with nitrone antibody. When mixtures of metHb (10 [mu]M as heme) and different concentrations of KP were irradiated with UVA, the formation of metHb radical nitrone adducts increased with increasing KP concentration and UVA dose (Fig. 1). At the lowest UVA dose (1 J/cm^sup 2^), UVA alone did not result in the formation of detectable metHb radical nitrone adduct; however, at higher doses of UVA alone (4 and 10 J/cm^sup 2^), there was increased production of adduct. When KP (25 or 200 [mu]M) was present, the metHb radical level increased significantly. The presence of 200 [mu]M KP induced a dramatic increase in metHb radical production. In the absence of the spin-trap DMPO, no radical nitrone adduct was detected (data not shown), indicating that the observed increase is due to the increased formation of radical nitrone adduct as a result of the reaction between the protein radical and DMPO.
Detection of metHb radical-derived nitrone adduct by Western blotting
To confirm the ELISA results, the metHb-KP-UVA-irradiated sample was subjected to Western blotting after exposure to the same primary and secondary antibodies used for ELISA (Fig. 2). When DMPO was omitted, no radical nitrone adduct was formed, demonstrating the specificity of the antiserum against hemoglobin-derived radical nitrone adducts in our Western blotting.
In the dark, KP alone up to 200 [mu]M did not induce the formation of metHb radical nitrone adduct as shown by the Western blot. When the reaction mixture was irradiated with UVA (4 and 10 J/cm^sup 2^), production of the nitrone adduct increased with increasing KP concentrations as well as increasing UVA doses, which is consistent with our ELISA results.
Role of hydrogen peroxide in the generation of the metHb radical nitrone adduct
Recently, Radschuweit et al. (16,17) have shown that hydrogen peroxide is generated during the UVA irradiation of an air-saturated aqueous solution of KP (16,17). Using the immuno-spin-trapping technique, we have detected the formation of metHb radicals on exposure of the protein to H^sub 2^O^sub 2^ (5,6). We, therefore, investigated the effect of catalase on KP-UVA-induced metHb radical formation and found that the adduct level decreased with increasing concentrations of catalase, reaching a plateau of 30% of control at 10 units/100 [mu]L and above (Fig. 3A). When DMPO was added after irradiation, the level of metHb radical nitrone adduct formation was similar to that observed when spin trap was present during irradiation (Fig. 3B), suggesting that protein radical formation was caused by the generation of a stable photoproduct. A decrease in adduct formation was observed when irradiation was carried out in the presence of nitrogen; however, under these conditions catalase did not produce any effect (Fig. 3B). Interestingly, in air-saturated solution the addition of SOD increased adduct formation by about 30% (data not shown). These findings show that H^sub 2^O^sub 2^ plays a key role in KP-UVA-induced metHb. We confirmed the generation of H^sub 2^O^sub 2^ using the Amplex Red assay and measured 39, 58 and 63 [mu]M H^sub 2^O^sub 2^ upon irradiation of 200 [mu]M KP with 4, 8 and 10 J/cm^sup 2^ UVA, respectively. Although the modification of tyrosine residues in metHb decreased adduct formation, derivatization of sulfhydryl groups was without effect (Fig. 4). Similar results were previously obtained when metHb was treated with H^sub 2^O^sub ^2 (6).
When KP was irradiated ([lambda] > 300 nm) in phosphate-buffered saline in the presence of DMPO, the electron paramagnetic resonance (EPR) spectrum shown in Fig. 5A was recorded. Analysis of the spectrum indicated that it contained contributions from the DMPO/O^sup *-^^sub 2^ (80%) and DMPO/^sup *^^OH (20%) adducts. In the presence of 100 [mu]M DTPA the intensity of the EPR spectrum increased (Fig. 5B) and consisted mainly (95%) of the DMPO/O^sup *-^^sub 2^ adduct. The addition of SOD abolished the DMPO/O^sup *-^^sub 2^ adduct spectrum (Fig. 5C), confirming that the adduct was indeed derived from the trapping of superoxide. In agreement with our previous findings (7) no adducts were detected when the solution was sparged with nitrogen before irradiation (Fig. 5D).
Comparison of metHb, metMb and oxyHb
As shown in Fig. 6, the replacement of metHb by an equimolar concentration of metMb did not result in any significant difference in nitrone adduct production. However, when metHb was replaced by an equimolar amount of oxyHb, the production of radical nitrone adduct decreased significantly. The finding that the ferric form of hemoglobin (metHb) generated more radical-derived nitrone adducts than the oxyferrous form (oxyHb) under the same experimental conditions is consistent with those of previous ESR spin-trapping (18) and immuno-spin-trapping studies (6).
Hemoglobin-derived nitrone adducts in RBC and hemolysate treated with UVA-KP
When an RBC suspension containing 10 [mu]M hemoglobin was irradiated with 10 J/cm^sup 2^ UVA in the presence of KP, the production of hemoglobin radical nitrone adduct increased significantly (Fig. 7A). A much smaller increase was observed in the absence of KP. However, at 4 J/cm^sup 2^ UVA the radical nitrone adduct was not observed until 200 [mu]M KP was present, in contrast to purified hemoglobin (Fig. 6), in which significant radical nitrone adduct was detected at 4 J/cm^sup 2^ in the absence of KP and much higher levels in the presence of 25 and 200 [mu]M KP. UVA at 10 J/cm^sup 2^ induced the formation of the hemoglobin nitrone adduct, which was enhanced by KP in RBC (Fig. 7A). When the reaction mixture shown in Fig. 7A was centrifuged, the hemoglobin nitrone adduct was detected only in the supernatant hemolysate (data not shown).
We have shown previously that KP-UVA causes RBC membrane damage and cell lysis (7,8). To determine whether cell lysis is required for hemoglobin radical formation, we measured KP-UVA-induced hemolysis (Fig. 7B) under the same conditions used for radical measurement (Fig. 7A). UVA alone at 10 J/cm^sup 2^ induced less than 10% hemolysis. In the presence of 25 [mu]M KP, UVA up to 4 J/cm^sup 2^ did not result in detectable hemolysis. The presence of 200 [mu]M KP resulted in a small amount of hemolysis after UVA of up to 4 J/cm^sup 2^. However, at 8 J/cm^sup 2^ UVA, cells were completely lysed by the presence of either 25 or 200 [mu]M KP, whereas at 4-8 J/cm^sup 2^ UVA, 25 [mu]M KP caused less hemolysis than 200 [mu]M KP. These results suggest that cell membrane damage and hemolysis precede hemoglobin radical formation.
When RBC were osmotically lysed before UVA-KP treatment, and the resultant total hemolysate (TH) was treated with UVA-KP, a significant increase in hemoglobin radical nitrone adduct was detected at 4 J/cm^sup 2^ in the absence of KP, whereas the presence of 200 [mu]M KP enhanced nitrone adduct formation dramatically (Fig. 8A). Removal of membrane debris from TH by centrifugation before UVA-KP treatment did not alter the formation of the hemoglobin radical nitrone adduct. However, after small non-protein antioxidant molecules had been removed from the centrifuged hemolysate by gel chromatography, radical nitrone adduct formation increased compared with the TH and the TH after centrifugation. This suggests that small nonprotein antioxidant molecules, such as ascorbate or GSH, can protect hemoglobin from protein radical formation. Furthermore, the addition of exogenous GSH (1 mM) inhibited hemoglobin radical nitrone adduct formation in RBC suspension treated with UVA-KP (Fig. 8B).
DISCUSSION
The photochemistry of KP relevant to the present study is shown in Scheme 2 (17,19-24). The key central intermediate is the carbanion (1) formed as a result of CO2 loss from KP. Reaction of 1 with oxygen generates the carbon-centered radical 2 and superoxide, which was trapped by DMPO (Fig. 5). Although 2 was not trapped by DMPO, we have previously detected this radical during the UV irradiation of KP by using 2-methyl-2-nitrosopropane as a spin trap (7). Dismutation of superoxide would give rise to H^sub 2^O^sub 2^, which then reacts with hemoglobin to generate the corresponding protein radical (5,6). The increase in the metHb radical adduct in the presence of SOD can be attributed to the rapid conversion of superoxide to H^sub 2^O^sub ^2 that occurs in the presence of the enzyme.
Although most of the metHb adduct generated by KP-UVA exposure could be attributed to the reaction of the protein with H^sub 2^O^sub 2^, approximately 30% was not affected by catalase (Fig. 3A). A similar level of adduct formation was observed when oxygen was excluded (Fig. 3B). These observations suggest that there are other pathway(s) to the metHb radical formation that do not involve H^sub 2^O^sub 2^. Under aerobic conditions, radical 2 (Scheme 2) may abstract a hydrogen atom from metHb. In the absence of oxygen, the KP photoproduct 3-ethylbenzophenone, 3, in its triplet state may be responsible for metHb radical formation (Scheme 2). Lahoz et al. (22) have proposed a similar mechanism for the UVA-induced covalent binding of KP to bovine serum albumin (22).
The protein radicals produced by KP-UVA treatment of hemoglobin were detected and determined by a validated antibody against the protein radical nitrone adduct (5). In our study, ELISA and Western blotting showed a consistent increase in metHb radicals after treatment with KP and UVA. It is evident that the protein radical levels were dependent on both UVA dose and KP concentration and that photogenerated H^sub 2^O^sub 2^ was mainly responsible for protein damage. UVA alone also induced the formation of protein radicals, which is most likely due to UVA photosensitization of the heme chromophore (25). Among the hemoglobins, oxyHb showed lower radical formation than metHb, indicating that redox status affects the photoreaction of KP and hemoglobins. Similar results have been observed during the reaction of hemoglobin with hydrogen peroxide (18).
Although KP photosensitized the formation of hemoglobin radicals in RBC, the protein radical level was much lower than that in purified hemoglobin. This may be due to at least two factors. First, the intact cell membrane provides a barrier against entry of KP, which is an anion at physiological pH. Second, although H^sub 2^O^sub 2^ photogenerated outside the erythrocytes should diffuse freely into the cells, it may be destroyed by intracellular antioxidants such as ascorbate and glutathione. The enhancement of radical formation after depletion of antioxidants (ascorbate and GSH) in the TH demonstrates that the production of hemoglobin radicals is inhibited by antioxidants as radical scavengers. As expected, the addition of GSH, which has been shown to protect against photohemolysis caused by KP (7), prevented the formation of protein radicals.
In summary, we have successfully demonstrated the detection of UVA-KP-sensitized hemoglobin protein radicals in both purified hemoglobin and RBC by the immuno-spin-trapping approach. The formation of protein radicals, which was mainly due to the generation of H^sub 2^O^sub 2^, was dependent both on UVA dose and KP concentration and was also affected by the iron redox status of the protein itself. These results are important for further understanding of the biological process involved in KP-induced photosensitization.
Acknowledgements-The authors are very grateful to Robert H. Sik for his technical assistance and Dr. Ann Motten for her critical reading of this manuscript.
[para]Posted on the website on 28 April 2003.
REFERENCES
1. Le Loet, X. (1989) Safety of ketoprofen in the elderly: a prospective study on 20,000 patients. Scand. J. Rheumatol. Suppl. 83, 21-27.
2. Mozzanica, N. and P. D. Pigatto (1990) Contact and photocontact allergy to ketoprofen: clinical and experimental study. Contact Dermatitis 23, 336-340.
3. Marguery, M. C., N. Chouini-Lalanne, J. C. Ader and N. Paillous (1998) Comparison of the DNA damage photoinduced by fenofibrate and ketoprofen, two phototoxic drugs of parent structure. Photochem. Photobiol. 68, 679-684.
4. Bagheri, H., V. Lhiaubet, J. L. Montastruc and N. Chouini-Lalanne (2000) Photosensitivity to ketoprofen: mechanisms and pharmacoepidemiological data. Drug Saf. 22, 339-349.
5. Detweiler, C. D., L. J. Deterding, K. B. Tomer, C. F. Chignell, D. Germolec and R. P. Mason (2002) Immunological identification of the heart myoglobin radical formed by hydrogen peroxide. Free Radic. Biol. Med. 33, 364-369.
6. Ramirez, D. C., Y.-R. Chen and R. P. Mason (2003) Immunochemical detection of hemoglobin-derived radicals formed by reaction with hydrogen peroxide: involvement of a protein-tyrosyl radical. Free Radic. Biol. Med. 34, 830-839.
7. Chignell, C. F. and R. H. Sik (1998) The effect of static magnetic fields on the photohemolysis of human erythrocytes by ketoprofen. Photochem. Photobiol. 67, 591-595.
8. Chignell, C. F. and R. H. Sik (1995) Magnetic field effects on the photohemolysis of human erythrocytes by ketoprofen and protoporphyrin IX. Photoehem. Photobiol. 62, 205-207.
9. Svistunenko, D. A. (2001) An EPR study of the peroxyl radicals induced by hydrogen peroxide in the haem proteins. Biochim. Biophys. Acta 1546, 365-378.
10. Gunther, M. R., R. A. Tschirret-Guth, H. E. Witkowska, Y. C. Fann, D. P. Barr, P. R. Ortiz De Montellano and R. P. Mason (1998) Site-specific spin trapping of tyrosine radicals in the oxidation of metmyoglobin by hydrogen peroxide. Biochem. J. 330 (Pt. 3), 1293-1299.
11. Witting, P. K., D. J. Douglas and A. G. Mauk (2000) Reaction of human myoglobin and H^sub 2^O^sub 2^. Involvement of a thiyl radical produced at cysteine 110. J. Biol. Chem. 275, 20391-20398.
12. Witting, P. K. and A. G. Mauk (2001) Reaction of human myoglobin and H^sub 2^O^sub 2^. Electron transfer between tyrosine 103 phenoxyl radical and cysteine 110 yields a protein-thiyl radical. J. Biol. Chem. 276, 16540-16547.
13. Winterbourn, C. C. (1990) Oxidative reactions of hemoglobin. Methods Enzymol. 186, 265-272.
14. Jeanmougin, M. and J. Civatte (1987) Dosimetry of solar ultraviolet radiation. Daily and monthly changes in Paris. Ann. Dermatol. Venereal. 114, 671-676.
15. Duling, D. R. (1994) Simulation of multiple isotropic spin-trap EPR-spectra. J. Magn. Reson. B. 104, 105-110.
16. Radschuweit, A., C. Huschka and H. H. Ruttinger (2000) Formation of hydrogen peroxide during the UVA induced disintegration of ketoprofen. Pharmazie 55, 782-783.
17. Radschuweit, A., H. H. Ruttinger, P. Nuhn, W. Wohlrab and C. Huschka (2001) UV-induces formation of hydrogen peroxide based on the photochemistry of ketoprofen. Photoehem. Photobiol. 73, 119-127.
18. McArthur, K. M. and M. J. Davies (1993) Detection and reactions of the globin radical in haemoglobin. Biochim. Biophys. Acta 1202, 173-181.
19. Bosca, F., M. L. Marin and M. A. Miranda (2001) Photoreactivity of the nonsteroidal anti-inflammatory 2-arylpropionic acids with photosensitizing side effects. Photoehem. Photobiol. 74, 637-655.
20. Bosca, F. and M. A. Miranda (1998) Photosensitizing drugs containing the benzophenone chromophore. J. Photoehem. Photobiol. B: Biol. 43, 1-26.
21. Bosca, F., M. A. Miranda, G. Carganico and D. Mauleon (1994) Photochemical and photobiological properties of ketoprofen associated with the benzophenone chromophore. Photoehem. Photobiol. 60, 96-101.
22. Lahoz, A., D. Hernandez, M. A. Miranda, J. Perez-Prieto, I. M. Morera and J. V. Castell (2001) Antibodies directed to drug epitopes to investigate the structure of drug-protein photoadducts. Recognition of a common photobound substructure in tiaprofenic acid/ketoprofen cross-photoreactivity. Chem. Res. Toxicol. 14, 1486-1491.
23. Martinez, L. J. and J. C. Scaiano (1997) Transient intermediates in the laser flash photolysis of ketoprofen in aqueous solutions: unusual photochemistry for the benzophenone chromophore. J. Am. Chem. Soc. 119, 11066-11070.
24. Miranda, M. A., J. V. Castell, D. Hernandez, M. J. Gomez-Lechon, F. Bosca, I. M. Morera and Z. Sarabia (1998) Drug-photosensitized protein modification: identification of the reactive sites and elucidation of the reaction mechanisms with tiaprofenic acid/albumin as model system. Chem. Res. Toxicol. 11, 172-177.
25. Batlle, A. M. (1993) Porphyrins, porphyrias, cancer and photodynamic therapy-a model for carcinogenesis. J. Photochem. Photobiol. B: Biol. 20, 5-22.
Yu-Ying He*, Dario C. Ramirez, Charles D. Detweiler, Ronald P. Mason and Colin F. Chignell
Laboratory of Pharmacology and Chemistry, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC
Received 23 December 2002; accepted 20 March 2003
*To whom correspondence should be addressed at: Laboratory of Pharmacology and Chemistry, National Institute of Environmental Health Sciences, National Institutes of Health, P.O. Box 12233, MD F0-06, Research Triangle Park, NC 27709, USA. Fax: 919-541-5750; e-mail: he3@niehs.nih.gov
Abbreviations: DMPO, 5,5-dimethyl-1-pyrroline N-oxide; DTPA, diethylenentriamine pentaacetic acid; ELISA, enzyme-linked immunosorbent assay; EPR, electron paramagnetic resonance; ESR, electron spin resonance; GSH, reduced glutathione; KP, ketoprofen; metHb, methemoglobin; metMb, metmyoglobin; NSAID, nonsteroidal anti-inflammatory drugs; oxyHb, oxyhemoglobin; RBC, red blood cells; SOD, superoxide dismutase; TH, total hemolysate; UVA, ultraviolet A.
Copyright American Society of Photobiology Jun 2003
Provided by ProQuest Information and Learning Company. All rights Reserved