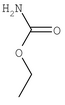
The modern era of golf ball covers was dominated by balata, a natural rubber that provided high spin rates and soft feel but lacked durability. It worked best with wound balls (the primary construction prior to 1970), because it adhered to windings well.
Surlyn and Iotek (copolymers developed by DuPont and Exxon) became available in the 1960s and were breakthroughs in cover technology. Spalding used Surlyn as the cover for the first two-piece balls. Surlyn is inexpensive, compared to balata, and cut-proof but feels hard.
The next breakthrough came when a softening agent was added to the copolymers. "People think Surlyn is a hard material," says Spalding's Tom Kennedy, "but by using these softening agents we can make an ionomer golf ball that has good scuff resistance and still has good spin. That was a big deal."
Urethane covers are another advancement. They are both durable and soft, yet somewhat costly, so are used mostly on high-end balls.
What's next? "The best thing would be a material that can mutate in flight," says Kennedy. "It would be one material when hit off the tee; as it moved through the air it would mutate to give low spin off the tee and high spin around the green."
COPYRIGHT 2001 New York Times Company Magazine Group, Inc.
COPYRIGHT 2001 Gale Group