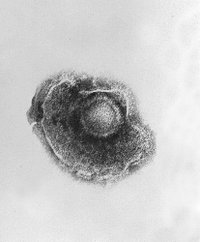
Varicella Zoster
The varicella-zoster virus (VZV), also known as human herpesvirus 3 (HHV-3), is one of the eight herpesviruses known to affect humans (and other vertebrates). Primary VZV infection results in chickenpox (varicella), which may rarely result in complications including VZV encephalitis. Even when clinical symptoms of varicella have resolved, VZV remains dormant in the nervous system of the host in the trigeminal and dorsal root ganglia. more...
In about 10-20% of cases, VZV reactivates later in life to produce herpes zoster (shingles) and its associated sequelae including: post-herpetic neuralgia, zoster multiplex, myelitis, herpes ophthalmicus, or zoster sine herpete.
VZV is closely related to the herpes simplex viruses (HSV), sharing much genome homology. The known envelope glycoproteins (gB, gC, gE, gH, gI, gK, gL) correspond with those in HSV, however there is not equivalent of HSV gD. VZV virons are spherical and 150-200 nm in diameter. Its lipid envelope encloses the nucleocapsid of 162 capsomeres arranged in a hexagonal form. Its DNA is a single linear, double strand molecule, 125,000 nt long.
The virus is very susceptible to disinfectants, notably sodium hypochlorite. Within the body it can be treated by a number of drugs and therapeutic agents including aciclovir, zoster immunoglobulin (ZIG), and vidarabine.
A live attenuated VZV Oka/Merck strain vaccine is available and is marketed under the trade name Varivax. It was developed by Merck, Sharp & Dohme in the 1980s from the Oka strain virus isolated and attenuated by Michiaki Takahashi and colleagues in the 1970s. It was submitted to the U.S. Food and Drugs Administration for approval in 1990 and was approved in 1995. Since then, it has been added to the recommended vaccination schedules for children in Australia, the United States, and many other countries, causing controversy because it is only expected to be effective for about twenty years, leaving adults vulnerable to the most dangerous forms of infection by this virus, whereas getting normal chickenpox as a child typically leaves them immune for life.
Read more at Wikipedia.org