The incidence of ventricular septal defect (VSD) occurs in up to 4.5% of penetrating cardiac trauma. We report a patient with persistent VSD who underwent surgical repair with significant left-to-right shunt and signs of heart failure. We performed a successful transcatheter closure of the VSD with an Amplatzer septal occluder (AGA Medical Corporation; Golden Valley, MN).
Key words: posttraumatic ventricular septal defect; transcatheter closure
Abbreviation: VSD = ventricular septal defect
**********
Review of the literature of penetrating cardiac trauma reports the incidence of ventricular septal defect (VSD) to be 4.5%. Most of patients are usually operated on because of heart failure and/or significant left-to-right shunt. We report a case report of a patient with penetrating chest trauma corrected by surgery, who subsequently developed heart failure due to a large persistent VSD. Successful transcatheter closure of the VSD was then performed with an Amplatzer septal occluder (AGA Medical Corporation; Golden Valley, MN).
CASE REPORT
A 40-year-old man, with no medical history, was stabbed with a knife in the anterior chest. He was hospitalized in a severe state of shock. After resuscitation, he was taken to the operating room. An emergency thoracotomy was done to relieve cardiac tamponade and to suture a right ventricular wound. Postoperatively, echocardiography revealed a large muscular VSD and significant tricuspid regurgitation due to a prolapsed septal valve. He was reoperated on the next day to close the VSD with a Teflon patch and repair two papillary muscles of the tricuspid valve because of significant regurgitation.
The postoperative course was uneventful. One month later, he presented short of breath with signs of heart failure. Echocardiography showed a large residual VSD with significant left-to-right shunting secondary to a dehiscence of the patch and tricuspid insufficiency grade 4/4. It was then planned to close the defect transvenously.
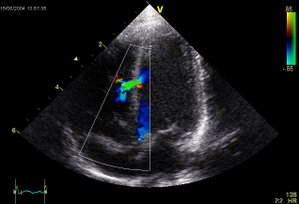
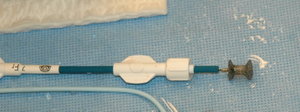
A cardiac catheterization was performed, and pressure records were as follows: left ventricle, 102/0 mm Hg; right ventricle, 35/0 mm Hg; pulmonary artery, 30/16/23 mm Hg; and fight atrium, 30/5/19 mm Hg. The left-to-right shunt was 3.7/1 by oximetry. A left ventricular angiography showed a 12-mm muscular VSD (Fig 1). We decided to close the defect with a 12-mm Amplatzer muscular VSD occluder. This device is made from Nitinol (1) wire mesh that has been shaped by heat treatment into a central stent with two retention discs. It is very similar to the atrial septal defect device. To place a 9F-delivery sheath within the left ventricle, an arteriovenous loop was first introduced from the arterial side with capture of 0.020-inch guidewire in the pulmonary artery. During placement of the occluder across the defect, the device slipped into the fight ventricle and was then retrieved within the delivery sheath. We decided thus to use a 15-mm Amplatzer septal occluder, which was similarly delivered (Fig 2). After release, control left ventricular angiography showed good position of the device and minimal residual shunt. On the next day, he presented with pallor and significant hematuria. Blood tests revealed intravascular hemolysis; his hemoglobin level fell from 14 to 8.5 g/dL, and haptoglobin level was 0.08 g/L. He did not require any blood transfusion, and hemolysis stopped spontaneously after a few days. Six months later, repeat echocardiography showed a tiny residual shunt and persistent tricuspid insufficiency. This patient is waiting for further surgery to repair the tricuspid valve.
DISCUSSION
According to literature, VSDs associated with cardiac trauma, either penetrating or blunt, (2) are uncommon but not rare. The mechanisms of VSD from blunt cardiac injury are believed to be either the acute compression of the heart between the sternum and the spine or rapid deceleration. VSD may here result from traumatic myocardial infarction due to an intimal tear of the coronary artery with thrombosis, spasm, embolism, dissection, or myocardial contusion. (3,4)
Cardiac injury following blunt trauma (3) is often unsuspected but may also produce instantaneous congestive heart failure due myocardial injury, VSD, and valvular injury. In most of the cases, VSD occurs in the muscular portion of the septum.
According to the literature, the decision to perform surgical repair is made on the amount of left-to-right shunt and presence of heart failure. (2,4) Transcatheter closure of a posttraumatic VSD can be an alternative to surgery in symptomatic patients with a systemic-to-pulmonary ratio > 1.5 following a blunt chest trauma or persistent shunt after surgical repair; however, a conservative approach has been recommended for small traumatic VSD with a pulmonary-to-systemic ratio < 2:1 because these defects may be well tolerated and close over time. More recently, some have reported transcatheter closure for perimembranous or muscular VSD using a new device, the Amplatzer VSD occluder. (1,5-8) In such cases, the VSD must not be too close to the aortic and atrioventricular valves to allow a safe procedure and no valvular device protrusion. No episode of hemolysis has been yet reported. We do think, according to the past experience in transcatheter patent ductus arteriosus closure, that hemolysis is related to high velocity jet through and around the occluder leading to destruction of RBCs. Usually, this resolved after complete closure of the defect by placement of a second occluder or simply after endothelialization of the device as was probably observed in our patient. To conclude, transcatheter closure appears to be a good option for patients beyond infancy with a posttraumatic muscular VSD that is hemodynamically significant.
REFERENCES
(1) Tofeig M, Patel RG, Walsh KP. Transcatheter closure of a mid-muscular ventricular septal defect with an Amplatzer VSD occluder device. Heart 1999; 81:438-440
(2) Olsovsky MR, Jopaz O, DiSciascio G, et al. Acute traumatic septal rupture. Am Heart J 1996; 131:1039-1041
(3) Engelman RM, Rousou JA, Schweiger M. Traumatic ventricular septal defect following closed-chest massage: a new approach to closure. Ann Thorac Surg 1984; 38:529-531
(4) Genoni M, Jenni R, Turina M. Traumatic ventricular septal defect. Heart 1997; 78:316-318
(5) Thanopoulos BD, Tsaousis GS, Konstadopoulo GN, et al. Transcatheter closure of muscular ventricular septal defects with the Amplatzer ventricular septal defect occluder: initial clinical applications in children. J Am Coll Cardiol 1999; 33:1935-1939
(6) Hijazi ZM, Hakim F, Al-Fadley F, et al. Transcatheter closure of single muscular ventricular septal defects using the Amplatzer muscular VSD occluder: initial results and technical considerations. Catheter Cardiovasc Interv 2000; 49:167-172
(7) Hijazi ZM, Hakim F, Haweleh AA, et al. Catheter closure of perimembranous ventricular septal defects using the new Amplatzer membranous VSD occluder: initial clinical experience. Catheter Cardiovasc Interv 2002; 56:508-515
(8) Chessa M, Carminati M, Cao QL, et al. Transcatheter closure of congenital and acquired muscular ventricular septal defects using the Amplatzer device. J Invasive Cardiol 2002; 14:322-327
* From the Departments of Pediatric Cardiology (Drs. Pesenti-Rossi, Godart, and Rey) and Cardiac and Vascular Surgery (Dr. Dubar), Cardiac Hospital, University of Lille, Lille, France.
Manuscript received July 11, 2002; revision accepted October 10, 2002.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (e-mail: permissions@chestnet.org).
Correspondence to: Francois Godart, MD PhD Service des Maladies Cardiovasculaires Infantiles et Congenitales Hopital Cardiologique, Bvd du Pr J. Leclercq, 59037, Lille cedex, France; e-mail: f-godart@chru-lille.fr
COPYRIGHT 2003 American College of Chest Physicians
COPYRIGHT 2003 Gale Group