Background: Recent data suggest that the risk of acquired ventricular septal defect (VSD), a complication of acute myocardial infarction (AMI), could be reduced using thrombolytic therapy. There are, however, still no available data regarding the potential impact of primary percutaneous coronary intervention (PCI) on AMI-related VSD in a clinical setting. The purposes of this study were to delineate the incidence and the potential risk factors of AMI-related VSD in the Chinese population, and to determine whether primary PCI could reduce such risk.
Methods and results: From May 1993 through March 2003, a total of 1,321 patients with AMI (for < 12 h) underwent primary PCI in our hospital. Of these 1,321 patients, 3 patients (0.23%) developed VSD after undergoing a primary PCI, with a mean ([+ or -] SD) time of occurrence of 25.3 [+ or -] 12.2 h. During the same period, a total of 616 consecutive, unselected patients with early AMI [ie, > 12 h and [less than or equal to] 7 days] or recent myocardial infarction (MI) [ie, [greater than or equal to] 8 days and < 30 days] who had not received thrombolytic therapy underwent elective PCI. Of these 616 patients, 18 (2.9%) had VSD either on presentation or during hospitalization, with a mean time of occurrence of 71.1 [+ or -] 64.2 h. Clinical variables were utilized to statistically analyze the potential risk factors. Univariate analysis demonstrated that the enrollment variables strongly related to this complication were advanced age, hypertension, nonsmokers, anterior infarction, female gender, and lower body mass index (BMI) [all p < 0.005]. Using multiple stepwise logistic regression analysis, the only variables independently related to VSD were advanced age, female gender, anterior infarction, and low BMI (all p < 0.05). The in-hospital mortality rate was significantly higher in patients with this complication than in patients without this complication (47.6% vs 8.0%; p < 0.0001). The incidence of this complication was significantly lower in patients with AMI who underwent primary PCI than in those with early or recent MI who underwent elective PCI (3.0% vs 0.23%, respectively; p = 0.0001).
Conclusion: Primary PCI had a striking impact on reducing the incidence of VSD after AMI compared to elective PCI in patients who did not receive thrombolytic therapy. Advanced age, female gender, anterior infarction, and low BMI had potentially increased the risk of this catastrophic complication after AMI in this Chinese population.
Key words: coronary angioplasty: myocardial infarction: ventricular septal rupture
Abbreviations: AMI = acute myocardial infarction; BMI = body mass index: GUSTO = Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries; MI = myocardial infarction; PCI = percutaneous coronary intervention; TIMI = thrombolyis in myocardial infarction; VSD = ventricular septal defect
**********
While ventricular septal defect (VSD) is an uncommon mechanical complication after acute myocardial infarction (AMI) and has a reported incidence during the prethrombolytic era of 1 to 2%, (1,2) it carries an extremely high mortality rate either with or without surgical intervention. (1-5) Previous studies (2,6-8) s have demonstrated that this complication typically occurs during the first week after AMI, usually with a mean time of 3 to 5 days from symptom onset. Investigators also have demonstrated that this complication occurs more often in patients of advanced age, (5) of female gender, (1,5,9,10) with total occlusion of the infarct-related artery, (5,10,11) and with absence of a previous MI. (1,12) The impact of thrombolytic therapy on AMI-related VSD had not been well-established until recently from the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO)-I trial. (5) Their study (5) demonstrated that thrombolytic therapy provided the additional benefits of reduced risk of AMI-related VSD. Surprisingly, the effects of primary percutaneous coronary intervention (PCI) on reducing the risk of AMI-related VSD has not been well-established.
Therefore, we analyzed the pooled data and compared patients with AMI (ie, < 12 h) who were undergoing primary PCI and patients with early myocardial infarction (MI) [> 12 h and [less than or equal to] 7 days] or recent MI ([greater than or equal to] 8 days and < 30 days) who had not undergone thrombolytic therapy but were undergoing elective PCI in our hospital, in order to delineate the baseline and clinical variables associated with this complication In addition, we tried to identify the prevalence of VSD after AMI in a Chinese population. Finally, the aim of this study was to evaluate the value of primary PCI in reducing the incidence of this complication.
MATERIALS AND METHODS
Patient Population
>From May 1993 through March 2003, primary, PCI was performed in 1,321 consecutive and unselective patients of any age who presented with AMI (ie, < 12 h). Of these 1,321 patients, 3 (0.23%) developed VSD after primary PCI. During the same period, a total of 616 consecutive unselected patients with early MI or recent MI who had not received thrombolytic therapy underwent elective PCI. Of these 616 patients, 18 (2,9%) were found to have VSD either on presentation or during hospitalization. Figure 1 illustrates the excluded and eligible patients for primary or elective PCI.
[FIGURE 1 OMITTED]
Procedure and Protocol
Before stents were available in our country, balloon angioplasty was performed in these patients, However, after stents were available in our country, stent implantation was strongly encouraged unless the infarct related artery had heavy calcification, a reference lumen diameter of < 2.5 mm, or a stent-like result at the treatment site after balloon angioplasty. Left ventriculograms, which were immediately performed after angioplasty, were recorded for 30[degrees] right anterior oblique and 60[degrees] left anterior oblique views.
Echocardiography was routinely performed in each patient either before or after undergoing cardiac catheterization. However, echocardiography was performed before cardiac catheterization when the presence of a heart murmur (observed by physical examination) complicating the MI was suggested.
The routine medical treatment of these patients was as follows. First, the patient was pretreated with aspirin (324 mg) and was given a bolus of 5,000 U heparin. This was followed by an IV heparin drip, which was adjusted to keep the activated partial thromboplastin time at 1.5 to 2 times the normal values. Before angioplasty, an additional bolus of heparin was given to achieve an activated clotting time of [greater than or equal to] 300 s. Tirofiban has been available in our country since August 2000. Therefore, it was given to 406 patients in our study. Patients were treated with ticlopidine (250 mg bid) for at least 2 weeks if stent deployment was performed. Aspirin (100 mg po daily) was administered indefinitely to each patient. All other medications, nitrates, [beta]-blockers, diuretics, and angiotensin-converting enzyme inhibitors were used as needed in the patients.
Definitions
AMI was defined as typical chest pain lasting for > 30 min with ST-segment elevation of > 1 mm in two consecutive precordial new interior leads, or typical chest pain lasting for > 30 min with a new onset of complete left bundle branch block. Early MI was defined as an AMI that developed at > 12 h and [less than or equal to] 7 days in a patient with a history of typical chest pain (with or without an elevation of creatine kinase levels and a creatine kinase-MB fraction of > 4%), and series changes in ECGs followed by echocardiographic and angiographic findings of regional hypokinesis of the left ventricle. Recent MI was defined as an AMI that developed at [greater than or equal to] 8 days and < 30 days in patients with a history of typical chest pain, and series changes in ECGs followed by echocardiographic and angiographic findings of regional hypokinesis of the left ventricle.
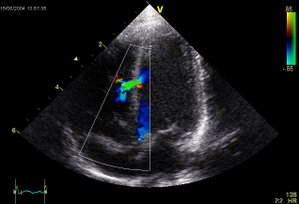
Body mass index (BMI) was defined as the weight in kilograms divided by the square of the height in meters (kg/[m.sup.2]). VSD was first suggested by physical examination findings. It was subsequently confirmed using echocardiography, and by a significant step-up of oxygen saturation between the right atrium and the pulmonary artery using a Swan-Ganz catheter, left ventriculogram, and operative findings. When VSD was found on presentation, we tried to review the source in medical records. Multivessel coronary artery disease was defined by a stenosis of > 50% in two or more major epicardial coronary arteries.
Data Collection
Detailed in-hospital and follow-up data, including age, sex, coronary risk factors, present or absent congestive heart failure on presentation, Killip score on hospital admission, reperfusion time, preintervention and postintervention thrombolysis in myocardial infarction (TIMI) flow grades, angiographic results, number of diseased vessels, and number in-hospital adverse events, were obtained. These data were collected prospectively and were entered into a computerized database.
Statistical Analysis
Data were expressed as the mean [+ or -] SD. Continuous variables were compared using the Wilcoxon rank sum test. Categoric variables were compared using the [chi square] test or Fisher exact test. Stepwise logistic regression analysis was used to determine independent determinants of VSD complicating AMI. Statistical analysis was performed using a statistical software package (SAS for Windows, version 8.9; SAS Institute; Cary, NC). A probability value of < 0.05 was considered to be statistically significant.
RESULTS
Baseline Clinical Characteristics of the Patients
Relevant patient baseline characteristics are summarized in Table 1. There were no significant differences in terms of the presence of diabetes mellitus, hypercholesterolemia, and previous MI between patients with and without VSD. However, patients with VSD had significantly higher incidences of hypertension, and more of these patients were nonsmokers than were patients without this complication. Furthermore, patients with VSD were significantly older and thinner than patients without this complication. Moreover, the incidence of occurrence in female patients was significantly higher in patients with VSD than in patients without it.
Clinical and Angiographic Findings and In-Hospital Mortality Rate
Relevant clinical and angiographic results and in-hospital mortality are summarized in Table 2. There were no significant differences in terms of pre-TIMI flow grades, presence of multivessel disease, or intercoronary collaterals between patients with and without VSD. However, the incidence of the left anterior descending artery as an infarct-related artery and the incidence of the total occlusion of the left anterior descending artery were significantly higher in patients with VSD than in patients without it. Furthermore, the incidences of cardiogenic shock and mortality rate were significantly higher in patients with VSD than in patients without it.
Operations were performed in the three patients who underwent primary PCI and later developed VSD. One patient died in the hospital due to multi-organ failure, and the other two patients were discharged from the hospital uneventfully and have survived to the present. Three of the 18 patients who had early or recent MI complicated by VSD refused operations and died in the hospital. The other 15 patients underwent the operation. Six of these 1,5 patients died in the hospital after their operation. The other patients were discharged from the hospital uneventfully. There was no statistically significant difference in in-hospital mortality between patients who had later VSD after primary PCI (33.3% [1 of 3 patients]) and patients with early or recent MI complicated by VSD (50% [9 of 18 patients]; p = 1.0). However, patients who had received medical treatment had a statistical tendency for a significantly higher in-hospital mortality rate (100% [3 of 3 patients]) than did patients who had received surgical treatment (38.7% [7 of 18 patients]; p = 0.09).
Independent Predictors of Ventricular Septal Rupture
Independent determinants of VSD are summarized in Table 3. Multiple stepwise logistic regression analysis of enrolled baseline and clinical variables demonstrated that only advanced age, female gender, anterior infarction, and low BMI were significant independent predictors of VSD after MI.
Timing of Occurrence and Incidences of Ventricular Septal Rupture Among Patients With AMI, Early MI, or Recent MI
AMI-related VSD developed in patients who underwent primary PCI at a mean time of 25.3 [+ or -] 12.2 h (range, 12 to 36 h). On the other hand, VSD developed in patients with early or recent MI who had not received thrombolytic therapy, and they underwent elective PCI at a mean time of 71.1 [+ or -] 64.2 h (range, 12 h to 12 days). The timing of the occurrence of VSD had a statistical tendency of significant shortening in patients with AMI who underwent primary PCI than in patients with early or recent MI who underwent elective PCI (p = 0.06). Univariate analysis demonstrated that the incidence of VSD in patients with AMI who underwent primary PCI was significantly lower (0.23% [3 of 1,321 patients]) than that in patients with early or recent MI who underwent elective PCI (2.9% [18 of 616 patients]; p < 0.0001).
DISCUSSION
Potential Impact of Primary PCI on AMI Complicated by Ventricular Septal Rupture
In the present study, one of the important findings was that, when compared with those patients who did not receive thrombolytic therapy who underwent elective PCI, primary PCI markedly reduced the incidence of AMI-related VSD. The results from the GUSTO-I trial (5) also demonstrated that thrombolytic therapy reduced the potential risk of AMI-related VSD. Our findings were consistent with the results from GUSTO-I trial. (5) Results from our present and recent studies, (13) and from other current data, (5,14-17) suggest the concept that early and complete restoration of the infarct-related artery by either thrombolytic therapy or primary PCI minimizes the effects of ischemic insult on the myocardium, preserves left ventricular function, reduces later VSD, and ultimately improves the overall survival of patients with AMI.
Incidence, Timing, Possible Mechanisms, and Predisposed Factors of Ventricular Septal Rupture Complicating AMI
In the present study, we found that the incidence of AMI-related VSD was 2.9% (18 of 616 patients) in our population of patients who did not receive thrombolytic therapy and underwent elective PIC. Our findings are comparable with those of previous studies from the prethrombolytic era (1,2) and suggested that the incidence of AMI-related VSD would have no racial differences between Chinese and western populations. (1,2)
It has been stated that VSD usually occurred at a mean time of 3 to 5 days from symptom onset in the prethrombolytic era.(2,6-8) In contrast, most VSDs associated with early thrombolytic therapy occur at a peak incidence time within 24 h after treatment. (5) The results of the present study demonstrated that the mean time of VSD was 3 days in those patients whose presentation was delayed and who underwent elective PCI. Our data were comparable with those of previous studies in the prethrombolytic era. (2,6-8) Additionally, in our study one valuable finding was that the mean time of occurrence of AMI-related VSD after primary PCI had a statistical tendency of being earlier than that of the elective PCI group. Our present findings were comparable with those from our recent report (13) regarding the timing of free wall rupture after AMI in those patients undergoing primary PCI and with those of other current studies, (1,2,5,18,19) which suggested that the time sequence of AMI-related VSD or free wall rupture in patients who had received various strategic treatments was different, as shown following thrombolytic therapy, mechanical reperfusion therapy (ie, primary PCI), and conventional therapy.
Previous studies (2,6-12,18-22) and our clinical observations have increased our understanding, leading us to speculate that the pathogenesis of a VSD may be a sequence of several mechanisms and preexisting predisposed factors acting together. First, an extensive and complete infarct area is a prerequisite for the cardiac rupture. This could be explained by the fact that after an MI, the infarct area usually showed a paradoxical motion during systole. This paradoxical motion was more vigorous in the larger infarct interventricular septal area, which in turn, led to a potential risk for rupture of the septum. This could explain that the incidences of left anterior descending artery infarctions and the angiographic findings that totally occluded left anterior descending artery were significantly higher in patients with VSD than in patients without VSD complicating AMI. (5) This could also explain why anterior placement of the MI was an independent determinant of VSD in the present study. Second, the synergistic effect of BP and expansion thinning of the interventricular septal area plays another important role in VSD. The results of a previous study (20) have demonstrated that infarct-area thinning occurs in patients with infarct expansion before resorption has occurred. The more extensive the infarction, the greater the expansion cavity dilatation of the left ventricle. Furthermore, high BP increased the wall tension, intramural stretching, and tearing of myocardial fibers during systole. Consequently, the left ventricular dilatation and wall remodeling were accelerated. The LaPlace law (ie, that wall stress is directly proportional to intracavity pressure and its radius, and is inversely proportional to wall thickness) may, at least in part, explain why AMI related VSD or free wall rupture was more likely, to occur in patients with a history of hypertension in previous studies (5,13,18) and in our present study. Third, we suggest that VSD complicating AMI may be due to an insult of "reperfusion injury." Successful reperfusion in the infarct-related artery would accelerate the rate of the inflammatory and healing processes, and would aggravate the absorption rate of necrotic tissue in the infarct area by increasing the transportation of inflammatory cells and proteolytic enzymes. This in turn, leads to thinning and softening of the necrotic zone, and to decreased tensile strength of the myocardial fibers. This could explain why VSD complicated by AMI usually, occurs earlier in patients who have undergone primary PCI than in the prethrombolytic era. Moreover, beyond those mechanisms mentioned above, several possible additional ones, including the extension of myocardial hemorrhage weakening and the dissection of the necrotizing zone, (18,19) the diminishing of myocardial collagen content, (23) and the digestion of collagen by collagenases (24,25) and plasmin, (26) also have been suggested to accelerate the occurrence of cardiac rupture during thrombolytic therapy. In other words, it is not surprising that AMI-related VSD would develop early in the course of thrombolytic therapy rather than during primary PCI reperfusion.
Advanced age has been found to be an increased risk factor for adverse outcomes after AMI. (5,13,27) The risks increased proportionally with advancing age, and primary angioplasty did not alter the relationship between adverse outcomes after AMI and being elderly. (27) In the present study, old age was found to be an independent predictor of AMI-related VSD. However, the cause of the high incidence of cardiac rupture in the elderly remains undefined and may perhaps be due to the disparity between left ventricular compliance in the aged and the age-related structural changes of the ventricular myocardium.
Morbidity, and mortality rates after AMI have been Reported (28) to be higher in women than in men. Recently, in patients with coronary artery disease undergoing PCI, lean patients were reported (29) to be at increased risk for in-hospital complications and cardiac death. In the present study, female gender and lower BMI were rote of the most important independent predictors of AMI-related VSD. The results of our study were consistent with those of other studies, (5,28,29) and suggested that female gender and lean body mass are universal risk factors for the occurrence of untoward cardiac: events during PCI.
Formidable, Challenges for Management of Ventricular Septal Rupture
Despite improvements in medical therapy and in percutaneous and surgical techniques, the mortality rate due to VSD complicating AMI remains extremely high. (5) In the present study, we found that more than half of the patients with VSD presented with cardiogenic shock and that 47.6% of the patients with this complication died in the hospital. Our findings were consistent with those in another report. (5) Although surgical intervention significantly improved the survival rate compared with conservative treatment in patients with this catastrophic complication, an in-hospital mortality rate of nearly 40.0% was observed after surgical intervention in the present study. This suggested that further therapeutic and management research is still required in patients with such a clinical condition.
There were several limitations to this study. First, the number of patients in the present study, although it is one of the largest series reported in the intervention era, was relatively small and had limited statistical power. Second, only three patients with AMI complicated by VSD were found in the primary PCI era. Therefore, the timing of the occurrence of AMI-related VSD had only a statistical tendency of significant shortening in patients with AMI undergoing primary PCI when compared with patients with early or recent MI undergoing elective PCI (p = 0.06), which could have been due to a type-2 error. Furthermore, the clinical symptoms and signs in patients with AMI-related VSD mostly had an insidious presentation. Progressive dyspnea, worsening congestive heart failure, and pansystolic heart murmur found during physical examination were the usual initial presentations. Therefore, a bias regarding the exact timing of the occurrence could not be completely ruled out. Finally, some patients may have died before arriving at our hospital or could not be diagnosed on presentation. Therefore, the true incidence of AMI-related VSD may have been underestimated in our study. Furthermore, the mean time of occurrence of VSD in these patients may be earlier than 71.1 h. Hence, caution should be used when extrapolating the results of this study for use as a reference in the management of patients in the same clinical setting.
In conclusion, our study demonstrated that the incidence of VSD complicating AMI was comparable between the Chinese and western populations. (1,2) VSD may be the consequence of several mechanisms acting together with predisposed factors, including hypertension, female gender, low BMI, advanced age, and anterior infarction. When compared with those patients who did not receive thrombolytic therapy and underwent elective PIC, primary PCI markedly reduced the incidence of AMI-related VSD.
REFERENCES
(1) Heitmiller R, Jacobs ML, Daggett WM. Surgical management of postinfarction ventricular septal rupture. Ann Thorac Surg 1986; 41:638-691
(2) Topaz O, Taylor AL. Interventricular septal rapture complicating acute myocardial infarction: from pathophysiologic features to the role of invasive and noninvasive diagnostic modalities in current management. Am J Med 1992; 93:683-688
(3) Davies RH, Dawkins KD, Skillington PD, et al. Late functional results after surgical closure of acquired ventricular septal defect. J Thorac Cardiovasc Surg 1993; 106:592-598
(4) Jones MT, Schofield PM, Dark JF, et al. Surgical repair of acquired ventricular septal defect: determinants of early, and late outcome. J Thorac Cardiovasc Surg 1987; 93:680-686
(5) Crenshaw BS, Granger CB, Birnbaum Y, et al. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. Circulation 2000; 101:27-32
(6) Bedynek J, Fenoglio J, McAllister H. Rupture of the interventricular septum as a complication of myocardial infarction. Am Heart J 1979; 97:773-781
(7) Edwards SB, Ewards WD, Edwards JE. Ventricular septal rupture complicating acute myocardial infarction: identification of simple and complex types in 53 autopsies hearts. Am J Cardiol 1984:54:1201-1205
(8) Moore CA, Nygaard TW, Kaiser DL, et al. Postinfarction ventricular septal rupture: the importance of location of infarction and right ventricular function in determining survival. Circulation 1986; 74:45-55
(9) Feneley MP, Chang VP, O'Rourke MF. Myocardial rupture after acute myocardial infarction: ten year review. Br Heart J 1989; 62:268-272
(10) Cheriex EC, de Swart H, Dijkman LW, et al. Myocardial rupture after myocardial infarction is related to the perfusion status of the infarct-related coronary artery. Am Heart J 1995; 129:644-650
(11) Skehan JD, Carey C, Norrell MS, et al. Patterns of coronary artery disease in post-infarction ventricular septal rupture. Br Heart J 1989; 62:268-272
(12) Pohjola-Sintonen S, Muller JE, Stone PH, et al. Ventricular septal and free wall rupture complicating acute myocardial infarction: experience in the multicenter investigation of limitation of infarct size. Am Heart J 1989; 117:809-816
(13) Yip HK, Wu CJ, Chang HW, et al. Cardiac rapture complicating acute myocardial infarction in the direct percutaneous coronary intervention reperfusion era. Chest 2003; 124:565-571
(14) The GUSTO Angiographic Investigators. The effects of tissue plasminogen activator, streptokinase, or both on coronary-artery patency, ventricular function, and survival after acute myocardial infarction. N Engl J Med 1993; 329:1615-1622
(15) Simes RJ, Topol EJ, Holmes DR, et al. Link between the angiographic substudy and mortality outcomes in a large randomized trial of myocardial reperfusion: importance of early and complete infarct artery reperfusion. Circulation 1995; 91:1923-1928
(16) Kern MJ, Moore JA, Aguirre FV, et al. Determination of angiographic (TIMI grade) blood flow by intracoronary Doppler flow velocity during acute myocardial infarction. Circulation 1996; 94:1545-1552
(17) Stone GW, Brodie BR, Griffin JJ, et al. Prospective, multi-center study of the safety and feasibility of primary stenting in acute myocardial infarction: in-hospital and 30-day results of the PAMI stent pilot trial; the Primary Angioplasty in Myocardial Infarction (PAMI) Stent Pilot Trial Investigators. J Am Coll Cardiol 1998; 31:23-30
(18) Becker RC, Gore JM, Lambrew C, et al. A composite view of cardiac rupture in the United States national registry of myocardial infarction. J Am Coll Cardiol 1996; 27:1321-1326
(19) Becker RC, Charlesworch A, Wilcox RG, et al. Cardiac rupture associated with thrombolytic therapy: impact of time to treatment in the Late Assessment of Thrombolytic Efficacy (LATE) study. J Am Coll Cardiol 1995; 25:1063-1068
(20) Schuster E, Bulkley BH. Expansion of transmural myocardial infarction: a pathophysiological factor in cardiac rapture. Circulation 1979; 60:1532-1538
(21) Bedynek J, Fenoglio J, McAllister H. Rupture of the interventricular septum as a complication of myocardial infarction, Am Heart J 1979; 97:773-781
(22) Slater J, Brown RJ, Antonelli TA, et al. Cardiogenic shock due to cardiac free-wall rupture or tamponade after acute myocardial infarction: a report from SHOCK trial registry. J Am Coll Cardiol 2000; 36:1117-1122
(23) Factor SM, Robinson TF, Dominitz R, et al. Alterations of the myocardial skeletal framework in acute myocardial infarction with and without ventricular rapture: a preliminary report. Am J Cardiovasc Pathol 1986; 1:91-97
(24) Charney RH, Takahashi S, Zhao M, et al. Collagen loss in the stunned myocardium. Circulation 1992; 85:1483-1490
(25) Whittaker P, Boughner DR, Kloner RA. Role of collagen in acute myocardial infarct expansion. Circulation 1991; 84: 2123-2134
(26) Peuhkurinen KJ, Risteli L, Melkko JT, et al. Thrombolytic therapy with streptokinase stimulates collagen breakdown. Circulation 1991; 83:1969-1975
(27) Holmes DR, White HD, Pieper KS, et al. Effect of age on outcome with primary angioplasty versus thrombolysis. J Am Coll Cardiol 1999; 33:412-419
(28) Vakili BA, Kaplan RC, Brown DL. Sex-based differences in early mortality of patients undergoing primary angioplasty for first acute myocardial infarction. Circulation 2001; 104:3034-3038
(29) Gruberg L, Weissman NJ, Waksman H, et al. The impact of obesity on the short-term and long-term outcome after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol 2002; 39:578-584
* From the Divisions of Cardiology (Drs. Yip, Fang, Yeh, Fu, and Wu) and Cardiovascular Surgery (Dr. Tsai), Chang Gung Memorial Hospital, Kaohsiung; and the Department of Biological Sciences (Dr. Chang), National Sun Yat-Sen University, Kaohsiung, Taiwan, ROC.
Manuscript received May 30, 2003: revision accepted November 6, 2003.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (e-mail: permissions@chestnet.org).
Correspondent to: Chiung-Jen Wu, MD, Division of Cardiology, Department of Internal Medicine, Chang Gung Memorial Hospital, Kaohsiung, 123 Ta Pei Rd, Niao Sung Hsiang, Kaohsiung Hsien, 83301, Taiwan, ROC; e-mail: tang@adm.cmgh.org.tw
COPYRIGHT 2004 American College of Chest Physicians
COPYRIGHT 2004 Gale Group