The Food and Drug Administration recently approved new devices to treat two forms of heart defects that may be present at birth. The defects, also known as congenital heart defects, involve holes in the heart called septal defects.
The devices are used to repair two types: atrial septal defects, which involve a hole between the top chambers of the heart, and ventricular septal defects, which involve a hole between the bottom chambers of the heart.
Some of these defects are small enough that they don't require treatment. But if a hole is big enough, it can cause pressure in the lungs to rise abnormally high. People with these defects may also experience fatigue and a deficiency of oxygenated blood. Or, the heart may be overworked because blood is circulating improperly. Over time, serious and life-threatening problems may result. People with such defects should be evaluated by an appropriately trained physician.
People born with such defects commonly require treatment, which has previously involved medications or surgery. "But these new catheter-based devices may provide an alternative to surgery in properly selected patients," says Stuart Portnoy, M.D., acting chief of the FDA's interventional cardiology devices branch.
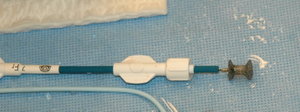
The CardioSEAL Septal Occlusion System, made by NMT Medical Inc. of Boston, was approved to close complex ventricular septal defects. The CardioSEAL was already approved for limited marketing under the agency's humanitarian device exemption, a special clearance that makes devices available for patients with rare medical conditions. Now, NMT Medical has approval for more widespread marketing for this indication.
The Amplatzer Septal Occluder, made by AGA Medical Corp. of Golden Valley, Minn., was approved to close certain atrial septal defects.
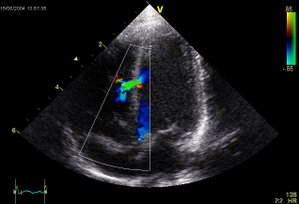
Made with either a metal frame or wire mesh and fabric, these occluders close the holes in the heart by covering the defect. Doctors make a small incision in either the groin or neck and thread a catheter through blood vessels to the site of the defect. Half of the occluder is carefully spread out, opening up somewhat like an umbrella. Then a sheath is pulled back through the hole to release the other half of the occluder. "The goal is to tightly sandwich the hole between both halves of the septal occluder," Portnoy says.
Regular X-rays and ultrasound are used to ensure that the patch stays in place. Over time, the heart's normal tissue develops on the occluder, which becomes part of the wall of the heart, permanently closing the defect.
Portnoy says, "Most children who need treatment for these types of heart defects are typically 5 years old or younger, and the new devices may help hundreds of them each year." Though the occluders are mostly used in children, there is no age restriction on their use and adults with these forms of congenital heart disease may also benefit.
After complaining of extreme fatigue that interfered with her daily activities, Sue Rosenbeck, 64, of St. Augustine, Fla., discovered she had two large holes in her heart. "When I thought surgery was the only option, I worried about how I would recover, especially because I have osteoporosis," she says. "I was so relieved to have another, less invasive option."
John P. Cheatham, M.D., director of cardiac catheterization and interventions at the Nemours Cardiac Center in Orlando, Fla., says he successfully treated Rosenbeck with two Amplatzer Septal Occluders. Rosenbeck was able to be discharged from the hospital less than 24 hours after the procedure. She says she notices a big difference in her energy level. She can keep up with her grandchildren now and has renewed energy for running her pet-sitting business.
"Appropriate patient selection is the number one criterion for safe use of these devices," Portnoy says. Individuals with these types of defects need to be carefully evaluated by physicians trained in the use of these devices.
"Potential risks with the devices include developing blood clots, infection, device fracture, or that the device could dislodge and be trapped inside the heart," Portnoy says. Fractures of the metal arms have occurred with the CardioSEAL device. No significant adverse events have been noted to date with the fractures.
The FDA is requiring that both NMT Medical Inc. and AGA Medical Corp. continue to study their products over the next five years to better assess their long-term safety and effectiveness.
COPYRIGHT 2002 U.S. Government Printing Office
COPYRIGHT 2004 Gale Group