Abstract
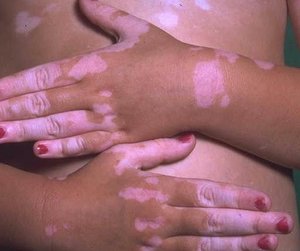
Vitiligo is a disfiguring skin disease. Many insights into its pathogenesis have been identified in recent years; however, treatment remains a challenge. In this article, the various treatment options for the treatment of vitiligo are outlined and newer treatment options are discussed.
Discussion
Vitiligo is a well-known cutaneous disease. Its basic pathology involves loss of melanocytes in areas of depigmentation. Its pathogenesis has been associated with genetic predisposition, autoimmune phenomenon, and neural and growth factor dysregulation, as well as inherent cellular metabolic defects leading to melanocyte apoptosis (for review, see footnote 1). Although strides have been made into identifying keys to the pathogenesis of vitiligo, treatment remains a challenge. Newer modalities and combination therapies have been studied, which give the clinician more treatment options and give patients hope for this psychologically devastating condition. This review will address some of the current and newer treatment options available to patients.
Current treatment options focus on four main categories: sunscreens, camouflage, depigmentation, and repigmentation. Sunscreens are not a therapy per se but do serve a central purpose in vitiligo therapy. They can minimize Koebner phenomenon secondary to sunburn seen in vitiligo, and they provide photoprotection of depigmented areas that have lost their innate sun protection from loss of melanocytes and hence melanin production. Broad spectrum sunscreens or sunblock to protect from UVA and UVB are recommended.
Camouflage is often used to "cover" affected areas. This may be practical for patients that have minimal disease or segmental disease. Many brand of dyes (Self-Sun Bronze Gel[TM] (Clinique), Dy-O-Derm[TM] (Galderma), Vitadye[TM] (ICN Pharmaceuticals)) or make-up are available. Some products are more cosmetically elegant than others. Patients need to be warned that these products may come off on clothing, or may need multiple applications during the day, especially in affected areas that are predisposed to frequent hand-washing such as the hands. Self-tanners such as Vitadye[TM], Dy-O-Derm[TM], and Chromelin[TM] (Summers Laboratories) contain dihydroxyacetone (DHA) which allows keratinocytes to chemically generate pigmentation that may help to blend affected areas. The mechanism of action of DHA occurs in the stratum corneum. The glycosidic hydroxyl group of DHA reacts with amino groups of keratin amino acids, peptides, and proteins in the skin. The resulting Maillard browning reaction produces melanoids. Color change can be seen in 20 minutes under a Wood's lamp and is clinically maximal in 8-24 hours. DHA-containing products should be applied only to depigmented lesions because they will darken normally pigmented skin and accentuate the color difference. The darkening is most apparent in areas of thicker stratum corneum, such as the palms, soles, knees, and elbows. However, the color match is not always exact and requires repeated applications to maintain color, which lasts for approximately seven days before the stratum corneum is sloughed off (1).
Depigmentation is a more drastic form of treatment available. It is an option for vitiligo patients with > 80% body surface area (BSA) involvement (i.e. vitiligo universalis). It is a permanent treatment and the result is full depigmentation of the skin. Monobenzylether of hydroquinone is the chemical used for this treatment. It is a bleaching agent with melanocyte cytotoxic activity although the exact mechanism of action remains obscure. It is applied as a 20% concentration on target areas twice per day. Depigmentation is often seen by one month but may take up to 6 to 12 months for full depigmentation. The major side effect includes irritant dermatitis but rarely limits therapy (3). A more recent method of depigmentation utilizes topical 4-methoxyphenol (4-MP) and the Q-switched ruby laser (QSR) (4). Up to 69% of patients in one study achieved depigmentation with 4-MP alone. Four out of five patients that did not respond to 4-MP responded to treatment with the QSR laser. Of note, some patients had recurrence of repigmentation. Side effects included localized burning or itching with 4-MP. No Koebner phenomenon was noted with laser treatments.
Repigmentation is the preferred treatment for patients with generalized vitiligo. Multiple modalities exist for repigmentation, including phototherapy, corticosteroids, surgical methods, laser therapy, and new agents.
Phototherapy is one of the oldest forms of treatment for vitiligo. As early as 1400 BC, Indian and Egyptian peoples were using psoralen-containing plant extracts and sunlight for repigmentation in vitiligo (5). Phototherapy options include psoralen with UVA (PUVA), UVB, and narrow-band UVB (NBUVB).
PUVA is the oldest form of modern phototherapy and remains the 'gold standard' for vitiligo treatment. The mechanism of action is believed to be stimulation of follicular and lesion border melanocytes that migrate to repopulate areas of depigmentation (6-8). Other studies have also shown that PUVA has suppressive effects on T cells and cytotoxic activity on mononuclear cells. This may result in immunosuppressant effects thereby inhibiting melanocyte destruction (7,9).
The indication for oral psoralen therapy with 8-methoxypsoralen is a BSA > 20% and for topical psoralen is BSA < 20%. Duration of therapy can be as long as 6 to 12 months. Average repigmentation rates are ~50% but can be higher in children, certain ethnic groups (African-Americans and Hispanics), and on certain locations such as the face and trunks. Side effects with topical PUVA include blistering and perilesional hyperpigmentation. Side effects with oral PUVA include nausea, vomiting, xerosis, lens opacities, and an increased risk of nonmelanoma skin cancers. However, prospective studies of vitiligo cohorts have failed to reveal an increase in skin cancer. In one recent study (10), a prospective study of 326 patients (children and adults) treated with oral and topical PUVA for a minimum of 4 months and followed for 4 years showed no increase in actinic keratoses, skin cancers, or lentigenes. These findings are unlike those seen in patients treated for psoriasis with PUVA and that may be because of lower mean total UVA doses used in vitiligo treatment. Although this study suggests no increased risk of skin cancer for vitiligo patients receiving PUVA, latency periods for skin cancers can be greater than 4 years thus, longer follow-up studies are needed to adequately address this potential risk.
Broad band UVB (BBUVB) has shown success in the treatment of vitiligo. The first report of such was in the 1990's (11). Mean repigmentation rates are ~57% and the treatment shows less toxicity compared to PUVA. However, the erythema produced by the shorter wavelengths of ultraviolet radiation (UV) make it a less desirable treatment method.
In recent years, narrow-band UVB (NBUVB, 311 nm) has become the preferred phototherapy for vitiligo. In fact, in the Netherlands, guidelines exist for NBUVB as first-line therapy in the treatment of adults with generalized vitiligo (12). The side effect profile is again superior over PUVA with no systemic toxicity, less erythema, pruritus, and xerosis, and overall less phototoxicity (13). Average repigmentation rates are ~63%, higher than that of PUVA or BBUVB. Also, NBUVB has been shown to be safe and effective in treating generalized vitiligo in children > 4 years of age (14). It has also been found safe to use in pregnant women (15). Length of treatment varies from 6 to 24 months and a maximum of 12 months is recommended in treating children. Treatments are well tolerated in both children and adults (15,16). Starting doses are usually 250-300 mJ/[cm.sup.2] for all skin types and the dose is increased by 10-20% until minimal erythema occurs at which time the dose is maintained. For facial involvement, we start at 100 mJ/[cm.sup.2] at our institution and increase by 25 mJ/[cm.sup.2] until minimal erythema. A recent prospective study compared the efficacy of treatment with NBUVB versus topical psoralen plus UVA. In this study (13), a total of 78 patients were treated with NBUVB and 28 patients received topical psoralen plus UVA for a duration of 4 months. Total body photographs from baseline and at the end of treatment were evaluated by blinded clinicians. The NBUVB group had higher repigmentation rates compared to the topical psoralen plus UVA group (67% vs. 46%). In a second part of this study, patients receiving NBUVB were followed for 1 year and showed an overall repigmentation rate of 63%.
Glucocorticosteroids have been used in the treatment of vitiligo and are often first-line therapy, especially in children or for localized disease. Although the mechanism of action is not entirely clear however, suppressive effects on both humoral and cellular immunity are presumed to be responsible for the effects. They can be used topically, intralesionally, or even systemically to halt actively progressing disease (2,17). The higher potency topical glucocorticosteroids have been shown to give good repigmentation results, however, their use is limited by localized side effects of skin atrophy, telangiectasias, hypertrichosis, and acne. Clinicians often will use 1-2 weeks of the high-potency steroid and alternate with a low to mid-potency steroid for 4-5 weeks to minimize side effects, or alternatively, treat with a mid-potency steroid alone. If no repigmentation is seen after 6 to 9 months of use, other treatment options should be considered (12). There have been recent studies showing that combination glucocorticosteroid topical therapy and phototherapy are more efficacious than either alone. In one study (18), researchers evaluated combination treatment with fluticasone propionate once daily and UVA twice a week and compared that to monotherapy with either fluticasone propionate alone or UVA alone. The results showed that combination therapy had higher repigmentation rates compared to either monotherapy.
Surgical treatments are another option for vitiligo. They are preferable in patients with stable disease or segmental vitiligo. Multiple modalities exist, including micropigmentation, grafting, and cultured autologous melanocyte transplantation.
Micropigmentation is a permanent treatment that involves iron oxide tattooing of a stable depigmented patch of vitiligo (19). Grafting is again suitable for stable patches and can be done in multiple ways using autologous punch grafts, thin split-thickness grafts, and suctions blister grafts (for review, see footnote 20). Repigmentation can take up to 6-9 months with grafting and the main adverse effects are scarring and uneven repigmentation.
Transplantation of cultured melanocytes was pioneered in the 1980's and various other techniques have been developed over the years (20-23). Briefly, the method involves harvesting autologous melanocytes from the donor site, culturing and expanding the melanocytes in special media, and then taking aliquots of the cultured melanocytes and implanting them in denuded areas of vitiligo. The technique gives an excellent color match but is expensive and involves complex culturing. Furthermore, cultured melanocytes grow poorly and require a medium supplemented with phorbol esters for proper expansion. What effect phorbol esters have on the malignant transformation of these melanocytes is unknown. Further in vitro and longer in vivo studies are needed to assess the spectrum of risks versus benefits. Laser therapy for vitiligo is a newer treatment option. One study evaluated the efficacy of the excimer (Xe-Cl, 308 nm) laser in treatment of vitiligo (23). A total of 3 treatments per week for 12 weeks were done. 57% of patients showed some repigmentation at the end of the study. Longer and larger studies are needed to assess the usefulness of this treatment in vitiligo. It appears that for localized and recalcitrant lesions or segmental vitiligo, this may be a useful treatment option. Another more recent study in thirty patients with segmental vitiligo employed the low-energy helium-neon laser (632.8 nm). In this study, 60% of patients had marked repigmentation (> 50%) after an average of 16 treatment sessions. In vitro studies done using the helium-neon laser on cultured keratinocytes showed increased production of nerve growth factor and basic fibroblast growth factor, factors known to stimulate melanocyte migration. Furthermore, melanocyte DNA synthesis and proliferation were stimulated with medium taken from irradiated keratinocytes. Melanocyte migration was stimulated both directly by irradiation with the helium-neon laser and indirectly using medium from irradiated keratinocytes (24). This study showed good clinical efficacy using a low-energy laser and proposed a biostimulatory effect on melanocytes as the mechanism of action.
Calcium modulators, the vitamin D3 analogues, have been used extensively in the treatment of psoriasis with good results. Recently, they have also been tried in the treatment of vitiligo. The rationale behind their use is based on in vitro data that has shown defective calcium transport in melanocytes and keratinocytes harvested from patients with vitiligo (25,26). Furthermore, vitamin D3 has been shown to activate melanin synthesis (27). An open study showed that the vitamin D3 analog calcipotriene was effective both as monotherapy and in combination with PUVA for the treatment of vitiligo (28). Another larger, controlled study from Turkey showed that combination therapy with calcipotriene and PUVA resulted in earlier repigmentation and lower total UVA doses compared to PUVA alone (29).
Calcineurin inhibitors/immunomodulators (tacrolimus, pimecrolimus) are the newest drugs in dermatology and have been approved for use in atopic dermatitis. These drugs have a unique mechanism of action. They bind the phosphatase calcineurin and inhibit the dephosphorylation of NFATc, a co-transcription factor important for transcription of cytokines IL-2, IL-3, IL-4, and TNF-[alpha]. Tacrolimus and pimecrolimus ultimately cause inhibition of these proinflammatory cytokines, thereby preventing activation of T cells. Unlike glucocorticoids which are also immunosuppressive, the immunomodulators do not cause inhibition of collagen synthesis therefore, they do not cause skin atrophy (30). Given the autoimmune hypothesis of vitiligo pathogenesis due to humoral and cellular dysfunction, the use of calcineurin inhibitors for the treatment of vitiligo seems reasonable. Recently, anecdotal reports of tacrolimus as successful monotherapy in the treatment of vitiligo have appeared (31). Most reports mention that facial lesions respond best and that spring and summer seasons have higher a success of repigmentation, likely secondary to concomitant sun exposure. Two small open studies have now been published and show tacrolimus to be efficacious as monotherapy for repigmentation in vitiligo (31-33). In one study (33), a total of 6 patients were treated with tacrolimus twice a day for a duration of 1-3 months. 1/6 had 100% repigmentation, and 4/6 had 50-75% repigmentation. Side effects were limited to temporary burning or stinging that resolved after 1-2 weeks.
Vitiligo poses a treatment challenge and will remain so until we find treatments that give consistent, long-term cure by repigmentation. However, newer therapies both as monotherapy and as combination therapy give hope that better treatments lie ahead. As further insights into the pathogenesis of vitiligo arise, other treatment options will come to light and should help us achieve better treatment outcomes for this disfiguring skin disease.
References
(1.) Fitzpatrick's Dermatology in General Medicine, 5th edition. Ed. Freedberg IM et al. New York, McGraw-Hill, 1999; pp 2750-51.
(2.) Njoo MD, Westerhof W. Vitiligo. Pathogenesis and treatment. Am J Clin Dermatol 2001; 2(3): 167-181.
(3.) Kovacs SO. Vitiligo. J Am Acad Dermatol 1998; 38(5 Pt 1):647-668.
(4.) Njoo MD, Vodegel RM, Westerhof W. Depigmentation therapy in vitiligo universalis with topical 4-methoxyphenol and the Q-switched ruby laser. J Am Acad Dermatol 2000; 42(5 Pt 1):760-769.
(5.) Grimes PE. Psoralen photochemotherapy for vitiligo. Clin Dermatol 1997; 15:921-926.
(6.) Morison WL. In vivo effects of psoralens plus long wave ultraviolet radiation on immunity. J Natl Cancer Inst 1984; 66:243-246.
(7.) Morison WL, Parrish JA, McAuliffe DJ, et al. Sensitivity of mononuclear cells to PUVA: Effect on subsequent stimulation with mitogens and on exclusion of typan blue dye. Clin Exp Dermatol 1981; 6:273-277.
(8.) Strauss GH, Bridges BA, Greaves M, et al. Inhibition of delayed hypersensitivity reaction in skin (DNCB test) by 8-methoxypsoralen photochemotherapy. Lancet. 1980; 2:556-559.
(9.) Toda K, Danno K, Tachibana T, et al. Effect of 8-methoxypsoralen plus long-wave ultraviolet radiation (PUVA) on mast cells: II. In vitro PUVA inhibits degranulation of rat peritoneal mast cells induced by compound 48/80. J Invest Dermatol 1986; 87:113-116.
(10.) Halder R, Battle EF, Smith EM. Cutaneous malignancies in patients treated with psoralen photochemotherapy (PUVA) for vitiligo. Arch Dermatol 1995; 131:734-735.
(11.) Koster W, Wiskermann A. Phototherapy with UV-B in vitiligo. Zeitschr Hautkrank 1990; 65:1022-24.
(12.) Njoo MD, Westerhof W, Bos JD, Bossuyt PMM. The development of guidelines for the treatment of vitiligo. Arch Dermatol 1999; 135:1514-21.
(13.) Westerhof W, Nieuweboer-Krobotova L. Treatment of vitiligo with UV-B radiation vs topical psoralen plus UV-A. Arch Dermatol 1997; 133:1525-28.
(14.) Njoo MD, Bos JD, Westerhof W. Treatment of generalized vitiligo in children with narrow-band (TL-01) UVB radiation therapy. J Am Acad Dermatol 2000; 42:245-53.
(15.) British Photodermatology Group. An appraisal of narrowband (TL-01) UVB phototherapy. British Photodermatology Group Workshop Report (April 1996). Br J Dermatol 1997 Sep; 137(3):327-30.
(16.) Scherschun L, Kim JJ, Lim HW. Narrow-band ultraviolet B is a useful and well-tolerated treatment for vitiligo. J AM Acad Dermatol 2001; 44:999-1003.
(17.) Radakovic-Fijan S, Furnsinn-Friedl AM, Honigsmann H, Tanew A. J Am Acad Dermatol 2001 May; 44(5):814-7.
(18.) Westerhof W, Nieuweboer-Krobotova L, Mulder PG, Glazenburg EJ. Left-right comparison study of the combination of fluticasone propionate and UV-A vs. either fluticasone propionate or UV-A alone for the long-term treatment of vitiligo. Arch Dermatol 1999 Sep; 135(9):1061-6.
(19.) Halder R, Pham H, Breadon J, et al. Micropigmentation for the treatment of vitiligo. J Dermatol Surg Onc 1989; 15:1092-98.
(20.) Halder RM, Young CM. New and emerging therapies for vitiligo. Dermatol Clinics 2000; 18(1):79-89.
(21.) Lerner AB, Halaban R, Klaus SN, Moellmann GE. Transplantation of human melanocytes. J Invest Dermatol 1987 Sep; 89(3):219-24.
(22.) Olsson MJ, Juhlin L. Repigmentation of vitiligo by transplantation of cultured autologous melanocytes. Acta Derm Venereol 1993 Feb; 73(1):49-51.
(23.) Spencer JM, Nossa R, Ajmeri J. Treatment of vitiligo with the 308-nm excimer laser: a pilot study. J Am Acad Dermatol 2002; 46(5):727-31.
(24.) Yu Hsin-Su, Wu Chieh-Shan, Yu Chai-Li, Kao Ying-Hsien, Chiou Min-Hsi. Helium-neon laser irradiation stimulates migration and proliferation in melanocytes and induces repigmentation in segmental-type vitiligo. J Invest Dermatol 2003; 120:56-64.
(25.) Schallreuter-Wood KU, Pittelkow MR, Swanson NN. Defective calcium uptake in keratinocyte cell cultures from vitiliginous skin. Arch Dermatol 1988; 280:137-9.
(26.) Schallreuter-Wood KU, Pittelkow MR, Swanson NN. Defective calcium transport in vitiliginous melanocytes. Arch Dermatol Res 1988; 288:11-13.
(27.) Abdl-Malek ZA, Ross R, Trinkle L, et al. Hormonal effects of vitamin D3 on epidermal melanocytes. J Cell Physiol 1988; 136:273-80.
(28.) Ameen M, Exarchou V, Chu AC. Topical calcipotriol as monotherapy and in combination with psoralen plus ultraviolet A in the treatment of vitiligo. Br J Dermatol 2001; 145:476-79.
(29.) Ermis O, Alpsoy E, Cetin L, Yilmaz E. Is the efficacy of psoralen plus ultraviolet A therapy for vitiligo enhanced by concurrent calcipotriol? A placebo-controlled double-blind study. Br J Dermatol 2001; 145:472-75.
(30.) Nghiem P, Pearson G, Langley RG. Tacrolimus and pimecrolimus: from clever prokaryotes to inhibiting calcineurin and treating atopic dermatitis. J Am Acad Dermatol 2002; 46:228-41.
(31.) Grimes, P. Therapeutic trends for the treatment of vitiligo. Cosmetic Dermatol 2002; 15(6):21-6.
(32.) Smith DA, Tofte SJ, Hanifin JM. Repigmentation of vitiligo with topical tacrolimus. Dermatology 2002; 205(3):301-3.
(33.) Grimes PE, Soriano T, Dytoc MT. Topical tacrolimus for repigmentation of vitiligo. J Am Acad Dermatol 2002 Nov; 47(5):789-91.
ADDRESS FOR CORRESPONDENCE:
Martha P Arroyo MD PhD
Ronald O. Perelman Department of Dermatology
560 First Avenue
New York, NY 10016
Email: mp.arroyo@stanfordalumni.org
COPYRIGHT 2003 Journal of Drugs in Dermatology
COPYRIGHT 2003 Gale Group