METHOD OF PREPARATION
Note: This preparation should be prepared in a laminar airflow hood in a cleanroom or via isolation barrier technology by a validated aseptic compounding pharmacist, using strict aseptic technique.
1. Calculate the required quantity of each ingredient for the total amount to be prepared.
2. Accurately weigh and/or measure each ingredient.
3. Mix the chloramphenicol, chlorobutanol, polyoxyl 40 stearate and polyethylene glycol 300 with about 90 mL of sterile water for injection and stir until dissolved.
4. Adjust the pH, if necessary, to the range of 7.0 to 7.5, using either sodium hydroxide or hydrochloric acid solutions.
5. Add sufficient sterile water for injection to volume and mix well.
6. Filter through an appropriate sterile 0.2-µm filter into sterile containers.
7. Package and label.
PACKAGING
Package in sterile ophthalmic containers.1
LABELING
Store in a refrigerator until dispensed. Keep out of reach of children. Use only as directed.1
STABILITY
A beyond-use date of up to 21 days can be used for this preparation after it is dispensed.1
USE
Chloramphenicol ophthalmic solution is indicated for the treatment of surface ocular infections caused by chloramphenicol-susceptible organisms2 involving the conjunctiva and/or cornea.
QUALITY CONTROL
Quality-control assessment can include weight/volume, physical observation, pH, specific gravity, osmolality, assay, clarity, particulate matter and sterility.3,4
DISCUSSION
Chloramphenicol 0.5% Ophthalmic Solution, which is no longer marketed, was formerly available from Allergan (Chloroptic) and Altana. It contained chloramphenicol, chlorobutanol, polyethylene glycol 300, polyoxyl 40 stearate, sodium hydroxide or hydrochloric acid and purified water.2 Chloramphenicol Ophthalmic Solution USP is a sterile solution of chloramphenicol containing not less than 90.0% and not more than 130.0% of the labeled amount of chloramphenicol. It has a pH in the range of 7.0 to 7.5, unless it is unbuffered, when the pH will be between 3.0 and 6.0.1
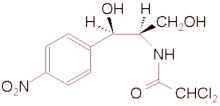
Chloramphenicol (C^sub 11^H^sub 12^C^sub 12^N^sub 2^O^sub 5^, MW 323.13) occurs as fine, white to grayish-white or yellowish-white, needlelike crystals or elongated plates. It is reasonably stable in neutral or moderately acidic solutions. It is slightly soluble in water (1 g in 400 mL) and freely soluble in alcohol and propylene glycol, acetone and ethyl acetate.1 It should be preserved in tight containers.1
Polyethylene glycol 300 (HOCH^sub 2^[CH^sub 2^OCH^sub 2^]^sub m^ CH^sub 2^OH, where m represents the average number of oxyethylene groups, MW 285 to 315, Carbowax, PEG, polyoxyethylene glycol) is an addition polymer of ethylene oxide and water. It occurs as a clear, colorless or slightly yellow-colored, viscous liquid with a slight, but characteristic odor and a bitter, slightly burning taste. It is soluble in acetone, alcohols, glycerin and glycols.5
Chlorobutanol (C^sub 4^H^sub 7^Cl^sub 3^O, MW 177.46) occurs as colorless or white crystals that are volatile and have a musty, camphoraceous odor. It is soluble in water (1 in 125), 95% ethanol (1 in 0.6) and glycerin (1 in 10). It is also freely soluble in volatile oils. It is classified as an antimicrobial preservative.6
Polyoxyl 40 stearate (C^sub 98^H^sub 196^O^sub 42^, MW 2046.61) occurs as a waxy solid that is off-white to light tan in color. It has a faint, bland, fatlike odor. It is soluble in alcohol and water and has a melting point of about 38°C. It is used as an emulsifying, solubilizing and wetting agent.7
Sterile water for injection is water for injection that has been sterilized and suitably packaged; it contains no added substances. Water for injection is water purified by distillation or reverse osmosis that contains no added substances. Water has a specific gravity of 0.9971 at room temperature, a melting point at 0°C and a boiling point at 100°C.1,8
REFERENCES
1. US Pharmacopeial Convention, Inc. United States Pharmacopeia 26-National Formulary 21. Rockville, MD:US Pharmacopeial Convention, Inc.; 2003:406-408, 1753, 2197-2201, 2554, 2589.
2. [No author listed.] Physicians' Desk Reference for Ophthalmology. 25th ed. Oradell, NJ:Medical Economics, Inc.; 1997:236-237.
3. Allen LV Jr. Standard operating procedure for particulate testing for sterile products. IJPC 1998;2:78.
4. Allen LV Jr. Standard operating procedure: Quality assessment for injectable solutions. IJPC 1999;3:406-407.
5. Price JC. Polyethylene glycol. In: Kibbe AH, ed. Handbook of Pharmaceutical Excipients. 3rd ed. Washington, DC:American Pharmaceutical Association; 2000:392-398.
6. Nash RA. Chlorobutanol. In: Kibbe AH, ed. Handbook of Pharmaceutical Excipients. 3rd ed. Washington, DC:American Pharmaceutical Association; 2000:126-128.
7. Yu CD. Polyoxyethylene stearates. In: Rowe RC, Sheskey PJ, Weller PJ, eds. Handbook of Pharmaceutical Excipients. 4th ed. Washington, DC:American Pharmaceutical Association; 2003:484-487.
8. Horry JM, Nash RA. Water. In: Kibbe AH, ed. Handbook of Pharmaceutical Excipients. 3rd ed. Washington, DC:American Pharmaceutical Association; 2000:546-549.
Copyright International Journal of Pharmaceutical Compounding Nov/Dec 2003
Provided by ProQuest Information and Learning Company. All rights Reserved