ABSTRACT
Trypsin and chymotrypsin are both serine proteases with high sequence and structural similarities, but with different substrate specificity. Previous experiments have demonstrated the critical role of the two loops outside the binding pocket in controlling the specificity of the two enzymes. To understand the mechanism of such a control of specificity by distant loops, we have used the Gaussian network model to study the dynamic properties of trypsin and chymotrypsin and the roles played by the two loops. A clustering method was introduced to analyze the correlated motions of residues. We have found that trypsin and chymotrypsin have distinct dynamic signatures in the two loop regions, which are in turn highly correlated with motions of certain residues in the binding pockets. Interestingly, replacing the two loops of trypsin with those of chymotrypsin changes the motion style of trypsin to chymotrypsin-like, whereas the same experimental replacement was shown necessary to make trypsin have chymotrypsin's enzyme specificity and activity. These results suggest that the cooperative motions of the two loops and the substrate-binding sites contribute to the activity and substrate specificity of trypsin and chymotrypsin.
INTRODUCTION
Serine proteases include a large class of enzymes. They provide much information on enzyme catalysis (1,2). Catalytic triad and oxyanion hole are important for enzyme activity of this category (3,4). These enzymes bypass the obstacles of breaking a peptide bond by properly positioning the catalytic triad (5), passing proton through them and forming catalytic intermediate (6,7), and stabilizing the tetrahedral intermediate with the oxyanion hole by electrostatic complementarities (8). Specificity is another aspect of enzyme catalysis. It is closely related to the enzyme-substrate interaction. From a mechanistic point of view, specificity is largely determined by the binding and the acylation step (2). Residues such as 189, 216, and 226 are important specificity determinants in these enzymes (9,10).
Hedstrom gave a thorough description in her recent review (2) about serine protease. Despite a long-time study, many aspects of this class of enzymes are still unclear. It is even not clear what the rate-limiting step in such proteases is. For poor amide substrates, acylation step seems to be rate limiting (11), whereas there is evidence that in serine protease like Kex2, deacylation step is rate limiting (12).
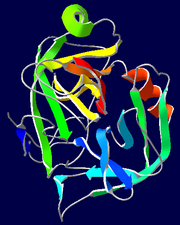
Trypsin and chymotrypsin are both serine proteases. The two enzymes have high sequence identity (13) and their tertiary structures are very similar (Fig. 1 A). In the chymotrypsin index, His-57, Asp-102, and Ser-195 form the catalytic triad, residues 189-195, 214-220, and 225-228 form the primary substrate-binding pocket called S1 binding pocket. Residues 185-188 and 221-224 form two loops near the S1 pocket, called L1 and L2, respectively (Fig. 1 B). Catalytic mechanisms of these two proteases are similar, but their substrate specificities are different. Trypsin favors basic residues like lysine and arginine; chymotrypsin favors aromatic residues like phenylalanine, tyrosine, and tryptophan (14). The S1 binding pocket in trypsin and chymotrypsin are almost identical in primary sequences and backbone tertiary structures (Fig. 1). An important difference is that residue 189 is a negatively charged Asp in trypsin and a polar Ser in chymotrypsin. This residue lies at the bottom of the S1 binding pocket and determines different S1 pocket chemical properties. This difference was once used to explain the different specificity of trypsin and chymotrypsin (15). But the mechanism is not that simple. Mutation of Asp-189 in trypsin (D189S) did not change the substrate specificity from trypsin-like to chymotrypsin-like (1,16,17); instead the enzyme just lost its activity. And mutation of S189D in chymotrypsin did not convert its specificity into that of trypsin, either (18). Comparison between the trypsin and trypsin mutant (D189S) shows little structural change in the S1 binding pocket (19). Hedstrom et al. showed that the S1 binding pocket only determines the specificity of ester hydrolysis, whereas specific amide hydrolysis requires both the proper S1 binding site and more distal interactions such as loops beside the substrate-binding pocket (1). When the two loops L1 and L2 of trypsin were replaced by those of chymotrypsin in addition to the D189S mutation, the new protein shows an increase of chymotrypsin activity to ~1000-fold against the D189S mutant (1). A site mutation not in contact with the substrate (Y172W) was found to improve the chymotrypsin-like activity of the hybrid protein by 20-50-fold (20). Gly-216 was also found to be a specificity determinant (21). The backbone conformation of Gly-216 differs between trypsin and chymotrypsin; but the hybrid enzyme adopts a chymotrypsin-like conformation (10,16,21,22). These experiments imply that in addition to the S1 substrate-binding pocket, loop regions of trypsin and chymotrypsin have significant effect on enzyme activity and substrate specificity.
Several explanations about the experiments on the specificity change have been proposed. An obvious one is from structure. The substitution of D189S deforms the S1 site and the activation domain (2,16,23). Mutations on L1 and L2 loops, and on Y172W may help to stabilize the S1 site (2,10). Though the specificity of chymotrypsin-like serine protease is usually categorized in terms of the P1-S1 interaction, a crucial feature of these proteases is that substrate occupancy of the S1 binding site alone confers only modest specificity (2). L1-L2 substitutions affect the conformation of Gly-216, which is an important residue to bind the P3 residue. Crystal structures show that the conformation of Gly-216 becomes chymotrypsin-like in the hybrid protein and help to orientate the scissile bond in the enzyme complex structure (21). The question remains as to how the L1-L2 substitutions change the conformation of Gly-216.
The above argument is from the static point of view. The other possibility is that the dynamical properties of the enzymes play an important role in the catalytic process. It is known in many cases that structure flexibility is closely related and crucial to the enzyme activity (24-27). A study of α-lytic protease has shown that plasticity of the substrate-binding pocket affects specificity of the enzyme (28). Studies on lipase showed that enzyme catalysis, substrate binding, and substrate releasing correspond to different types of motion styles (29). Enzyme loop regions have been shown to be important in catalysis (1,30-35). For the trypsin-chymotrypsin system, it is possible that certain modes of motion are essential for chymotrypsin catalysis, which can be influenced by the L1 and L2 loops. If only the trypsin S1 pocket is changed into chymotrypsin-like, it is not sufficient to change the specificity; but when L1 and L2 are also changed, global dynamics of the protein, may change to benefit the catalysis.
In this study, we have used the Gaussian network model (GNM) (36) and a clustering method to analyze the dynamic properties of trypsin and chymotrypsin. We find that the two enzymes have certain key differences in their dynamic motion. In particular, they differ in ways that the motion of the S1 binding pocket correlates with that of the loops L1 and L2, and with the nearby regions. When the two loops in trypsin are replaced with those of chymotrypsin, the hybrid enzyme vibrates in a similar way as chymotrypsin in some key parts. Taken together with experimental findings (1,21,37), our results suggest that the concerted motions of loop regions with the S1 binding pocket and the correlations between different binding sites can be important for the enzyme specificity.
MATERIALS AND METHODS
Gaussian network model
The Gaussian network model is a simplified model for normal mode analysis of proteins (36), in which a protein is converted into nodes connected by springs. All the nodes are identical and each of them represents a single residue. We use C^sub α^ atoms as the nodes in this study. All the nodes within a given distance r^sub c^ have interactions with each other. The connection here is simplified as harmonic force, with the same force constant. The distance of r^sub c^ is defined as 7 [Angstrom]. This value comes from the results of statistical analysis (38,39). All other atomic and structural details are ignored. This coarse-grained model was successfully used to reproduce the B-factors in x-ray diffraction experiment (40) and NMR experiment (41), to find kinetically hot residues (42), and to study relationships between slow vibration modes and the protein function (36,43,44).
Correlation analysis
Once we have the correlation matrix C^sub ij^, one way to use the matrix is to plot the matrix on a two-dimensional map, just like Fig. 2. This plot has been used in several studies (44,49-52). However, this map can only make clear correlations within and between big cliques of consecutive residues. Here we analyze the data in an alternative way. We change the correlation map into a distance map, and use clustering methods to analyze it. Similar procedures have been widely applied in genetic evolutionary analysis (53,54).
The crystal structure coordinates for bovine α-chymotrypsin (56) (Protein Data Bank (PDB) code: 4CHA) and bovine trypsin (J. A. Chamorro Gavilanes, J. A. Cuesta-Seijo, and S. Garda-Granda, unpublished data; PDB code: IS0Q) are used in this study.
RESULTS AND DISCUSSIONS
Correlation map
The correlation map C^sub ij^ (Eq. 5) of chymotrypsin is shown in Fig. 2. A number of features are evident. First, there are two highly correlated small squares at the diagonal around residues 160 and 235, respectively; these squares correspond to the two α-helices in chymotrypsin. The motions of the residues within each helix are highly correlated, implying that the α-helix is a compact and relatively independent structure motif with its own coherent motions (57). Second, there are several short lines of high correlation across and perpendicular to the diagonal (40-50, 57-62, 70-80, 100-110, 128-138, 142-152, 170-180, 192-197, 210-220). These correspond to β-sheets in the protein structure. Note that the correlation map shows certain information about the secondary structures though the model itself does not contain secondary structure information explicitly. Thirdly, there are two large weakly correlated regions in the bottom left (10-120) and top right (125-155, 175-220) of the map. These two regions correspond to the two β-barrels of chymotrypsin. No other large correlated movement can be seen from the map. In chymotrypsin, the smallest correlation is ~-0.1 and in other systems like HIV reverse transcriptase (49) the correlation could be more negative. This negative correlation is related to the specific structure and functional motion of the proteins. HIV reverse transcriptase is composed of many domains; motion between domains is functionally important. However, besides structural reasons, the correlations in the article of Bahar et al. (49) were enhanced because they only used the first four modes. If more modes are used, there will be more local fluctuations that do not contribute to the domain-domain correlation and due to the normalization with more modes their correlation values will be smaller. It is important to note that the mode number has a different effect on the maximum value of positive and negative correlations. The positive correlations exist among nearby residues; they often have the same motion style in most modes (especially the self-correlation), so that the mode number will not affect the positive correlation much. But the negative correlation can not exist among nearby residues; they will be affected by the mode number. Trypsin and chymotrypsin are relatively "stiff" enzymes; they do not have very long loops and also we use all the modes here so there are no big negative correlations.
Clustering analysis
After clustering the distance matrix of the pairwise correlations (Eq. 6), we obtain a tree map in which highly correlated residues cluster together (Fig. 3 B). These clusters provide dynamical information of the protein structure in addition to the traditional static view of protein domains, which may be functionally relevant. In Fig. 3 C several clusters are shown on the three-dimensional structure of chymotrypsin. Different clusters are painted with different colors. We can see that both L1 and L2 are located in the purple region together with residues in the S1 pocket Ser189, Ser-214, Trp-215, Gly-216, and Gly-226. Ser-189, Gly-216, and Gly-226 help define a deep hydrophobic pocket with other residues in chymotrypsin. Residues 214-216 have interactions with the P1-P3 residues of a peptide substrate.
Next we focus on the local tree branch near the L1-L2 loops of chymotrypsin in Fig. 4 A. In this figure, residues in the L1-L2 loops (shown as solid circles) and some residues in the substrate-binding pocket (solid triangles) are clustered together, so they move coherently. For trypsin, we also run this procedure and get a similar clustering map, which is shown in Fig. 4 B. Residues in the L1-L2 loops and several residues in the S1 binding pocket also cluster together, but the topology of the tree has changed. One obvious change is that in chymotrypsin, residues on the lid of the S1 pocket (217, 218, and 219) correlate with the L1 and L2 loops stronger than those in trypsin. We have known from experiments that loop replacement helps to change trypsin specificity to chymotrypsin specificity (1). Here we do the same experiment in silico by replacing the loops of trypsin with the loops of chymotrypsin. L1 structure of this hybrid protein is not known, but the backbones of the L2 loop in hybrid protein and chymotrypsin are similar (21). We assume that the configurations of the L1 loop do not change much from chymotrypsin to the hybrid protein. Because GNM is a coarse-grained method, it is reasonable to replace these regions directly after structure superposition (we changed the L1-L2 loops and 217-219). Fig. 4 C shows the local tree map for the hybrid protein by using the first 40 modes in the calculation. We see that the L1-L2 loops move coherently with several residues in the S1 binding pocket, just like in chymotrypsin. In particular, the lid of the pocket (217-219) clusters with the L1-L2 loops closely. In the hybrid protein, we get similar dynamic performance as in chymotrypsin. It is noteworthy that residue 138, 184-186, 188-189, 192, 217, and 221-224 in trypsin were mutated (1) in the experiment (Fig. 1B). Most of them can be found in one big branch of the tree-at least 13 in 15 of these resides appear together in the big branch for trypsin (Fig. 4 A), nine in 15 for the hybrid protein (Fig. 4 C). This may imply that these residues cooperate with each other to fulfill their function.
Mode analysis
To see how the loop motion influences the dynamics of the whole protein, we use only the most important modes for the loop motion listed above for the three proteins to calculate the residue fluctuations of the entire protein (Fig. 6 B). It is clear that after the loop substitution, fluctuations of the hybrid protein become similar to chymotrypsin, although it still has a trypsin backbone. The most obvious example comes from residues 85-105, which are not in the two loop regions, where in chymotrypsin there is big fluctuation and in trypsin the fluctuation is small. When the loops of trypsin are changed into that of chymotrypsin's, a peak appears in this region, showing that these residues have collective motions with the loops of chymotrypsin that are being placed in the hybrid protein. It is notable that one of the catalytic residues, Asp-102, and the essential residues Leu-99 for the S2-S4 substrate-binding sites are in this region. The different dynamical relationships between the two loops and these sites in trypsin and chymotrypsin may have functional implications on the two different enzymes.
Fig. 6, C and D, show some of the important modes we have identified. Mode 3 shown in Fig. 6 C is a common mode that has big contribution in all the proteins. Mode 11 in chymotrypsin, mode 10 in the hybrid protein, and mode 9 in trypsin are shown in Fig. 6 D. Mode 3 is similar in all of these proteins. Modes shown in Fig. 6 D are also similar in the loop region (190-194, 221-224). But in the region of residues 100-130, the mode of chymotrypsin and the hybrid protein are similar. In the region of residues 170-180, the mode of trypsin and the hybrid protein are similar. Although there are similarities and differences, a single mode cannot explain the correlation change of residue pairs that Figs. 5 and 6 B have shown. Several modes work together to change the relationship of residue pairs.
Correlation plot
To get a detailed and more direct picture of the residue correlations, we "plot" the correlation directly onto the three-dimensional structure. We use lines between two residues to illustrate the correlation between them (Fig. 7). Only large correlations (>0.6) are shown with lines. We also omit the correlations if the distance between two residues is
Conservation analysis
We extract 13 complete sequences of chymotrypsin and 64 sequences of trypsin from the ExPASy database (64). The sequence alignment was done using CLUSTAL_X (65) and the results are summarized in Table 1. The two loops are shown in black rectangles in Fig. 1 B. We notice that in both enzymes the length of Loop 1 is not conserved and the length of Loop 2 is conserved. In trypsin, L1 ranges four to seven residues in length and L2 is five residues in length. In chymotrypsin, L1 ranges four to five residues and L2 is four residues in length. The conservation of the length of L2 within chymotrypsin and trypsin may be important to the enzymes' selectivity. Previous experiments support this idea. In the experiment converting trypsin to chymotrypsin, trypsin with S1+L2 exchange is more active than the S1+L1 mutant (1). This means that L2 plays a more important role than L1. Compared with Ll, L2 is shorter in most cases and not so extended, especially in chymotrypsin. L2 links with the lid of the S1 pocket, which is also a flexible component of the protein; thus, transforming the motion of L2 to the S1 pocket is easier than that of L1. If we calculate the correlation between the S1 binding pocket and the L1 and L2 loops, we find the average correlation of L2-S1 is slightly stronger than the L1-S1 correlation (~0.01 times stronger).
Dynamic property of loops and the substrate specificity of enzyme reaction
Correlation analysis shows that the motions of the two loops and the substrate-binding pocket are highly correlated. The correlation between L1 and L2 in trypsin is mainly controlled by two major modes, whereas in chymotrypsin there are five major modes. Loop motion of L1-L2 affects the dynamical relationship of S1 and Loop D. The lengths of L1-L2 show very different conservations, which may be one of the reasons that L1 and L2 have different effects on enzyme specificity. When trypsin was mutated at the S1, L1, and L2 sites to those of chymotrypsin, the hybrid protein shows chymotrypsin-like loop correlations. All the evidence implies that the dynamic property of the two loops play a critical role in making trypsin and chymotrypsin different. This is in good accordance with the experiment (1) that shows that loop regions help to decide the specificity of chymotrypsin and trypsin. Miller and Agard (28) also reached the conclusion from a normal mode analysis that dynamics can be the determinant of substrate specificity in α-lytic protease. They found that the specificity of α-lytic protease correlates with the movement of the binding pocket. Molecular dynamics simulations also revealed the importance of the L1 and L2 loops in chymotrypsin catalysis: Wroblowski et al. showed that in both the activation and the deactivation of α-chymotrypsin, the targeted molecular dynamic path starts with a movement of Loop 2, pulling on Loop 1 (66). Both molecular dynamics simulation and the modified GNM model have revealed that sites that are spatially distant from active sites can have a strong mechanical influence on the structural modulation of the substrate-binding regions in HIV-1 protease (48).
CONCLUSIONS
We have studied the dynamical properties of trypsin and chymotrypsin and their relationship with enzyme specificity by using the Gaussian network model. A clustering method is introduced to analyze the correlations of the residues' motion. The two loops in trypsin and chymotrypsin were shown to have different dynamic properties that affect the correlations between other key sites in the two enzymes. When the two loops in trypsin were changed into chymotrypsin loops, the hybrid protein shows chymotrypsin-like cooperatvity. Our results suggest that chymotrypsin-like motions are important to the specificity of chymotrypsin. Changing the trypsin loops into chymotrypsin loops alters the motion style and, hence, the specificity.
SUPPLEMENTARY MATERIAL
An online supplement to this article can be found by visiting BJ Online at http://www.biophysj.org. The material includes the coordinates of the hybrid protein with the reconstructured loops.
This work was supported by the State Key Program of Basic Research of China (2003CB715900), the National Natural Science Foundation of China (90103029, 20173001, 20228306, 90403001, 30490240), the High-Tech Program of China, and the U.S. National Science Foundation (DMR 0313129).
REFERENCES
1. Hedstrom, L., L. Szilagyi, and W. J. Rutter. 1992. Converting trypsin to chymotrypsin: the role of surface loops. Science. 255:1249-1253.
2. Hedstrom, L. 2002. Serine protease mechanism and specificity. Chem. Rev. 102:4501-4524.
3. Blow, D. M. 1997. The tortuous story of Asp... His... Ser: structural analysis of alpha-chymotrypsin. Trends Biochem. Sci. 22:405-408.
4. Henderson, R. 1970. Structure of crystalline alpha-chymotrypsin. IV. The structure of indoleacryloyl-alpha-chymotrypsin and its relevance to the hydrolytic mechanism of the enzyme. J. Mol. Biol. 54:341-354.
5. Knowles, J. R. 1991. Enzyme catalysis: not different, just better. Nature. 350:121-124.
6. Hartley, B. S., and B. A. Kilby. 1954. The reaction of p-nitrophenyl esters with chymotrypsin and insulin. Biochem. J. 56:288-297.
7. Hengge, A., and R. Hess. 1994. Concerted or stepwise mechanisms for acyl transfer-reactions of p-nitrophenyl acetate: transition-state structures from isotope effects. J. Am. Chem. Soc. 116:11256-11263.
8. Matthews, D. A., R. A. Alden. J. J. Birktoft, S. T. Freer, and J. Kraut. 1975. X-ray crystallographic study of boronic acid adducts with subtilisin BPN' (Novo). A model for the catalytic transition state. J. Biol. Chem. 250:7120-7126.
9. Perona, J. J., and C. S. Craik. 1997. Evolutionary divergence of substrate specificity within the chymotrypsin-like serine protease fold. J. Biol. Chem. 272:29987-29990.
10. Perona, J. J., and C. S. Craik. 1995. Structural basis of substrate specificity in the serine proteases. Protein Sci. 4:337-360.
11. Butt, Z., P. M. B. Richard, and L. B. Myron. 1964. Kinetic evidence for the formation of acyl-enzyme intermediates in the α-chymotrypsin-catalyzed hydrolyses of specific substrates. J. Am. Chem. Soc. 86: 3674-3679.
12. Rockwell, N. C., and R. S. Fuller. 2001. Direct measurement of acylenzyme hydrolysis demonstrates rate-limiting deacylation in cleavage of physiological sequences by the processing protease Kex2. Biochemistry. 40:3657-3665.
13. De Haen, C., H. Neurath, and D. C. Teller. 1975. The phylogeny of trypsin-related serine proteases and their zymogens. New methods for the investigation of distant evolutionary relationships. J. Mol. Biol. 92: 225-259.
14. Vajda, T., and T. Szabo. 1976. Specificity of trypsin and alpha-chymotrypsin towards neutral substrates. Acta Biochim. Biophys. Acad. Sci. Hung. 11:287-294.
15. Steitz, T. A., R. Henderson. and D. M. Blow. 1969. Structure of crystalline alpha-chymotrypsin. 3. Crystallographic studies of substrates and inhibitors bound to the active site of alpha-chymotrypsin. J. Mol. Biol. 46:337-348.
16. Hedstrom, L. 1996. Trypsin: A case study in the structural determinants of enzyme specificity. Biol. Chem. 377:465-470.
17. Graf, L., A. Jancso, L. Szilagyi, G. Hegyi, K. Pinter, G. Naray-Szabo, J. Hepp, K. Medzihradszky, and W. J. Rutter. 1988. Electrostatic complementarity within the substrate-binding pocket of trypsin. Proc. Natl. Acad. Sci. USA. 85:4961-4965.
18. Venekei, I., L. Szilagyi, L. Graf, and W. J. Rutter. 1996. Attempts to convert chymotrypsin to trypsin. FEBS Lett. 379:143-147.
19. Perona, J. J., L. Hedstrom, R. L. Wagner, W. J. Rutter, C. S. Craik, and R. J. Fletterick. 1994. Exogenous acetate reconstitutes the enzymatic activity of trypsin Asp189Ser. Biochemistry. 33:3252-3259.
20. Hedstrom, L., J. J. Perona, and W. J. Rutter. 1994. Converting trypsin to chymotrypsin: residue 172 is a substrate specificity determinant. Biochemistry. 33:8757-8763.
21. Perona, J. J., L. Hedstrom, W. J. Rutter, and R. J. Fletterick. 1995. Structural origins of substrate discrimination in trypsin and chymotrypsin. Biochemistry. 34:1489-1499.
22. Varallyay, E., Z. Lengyel, L. Graf, and L. Szilagyi. 1997. The role of disulfide bond C191-C220 in trypsin and chymotrypsin. Biochem. Biophys. Res. Commun. 230:592-596.
23. Szabo, E., Z. Bocskei, G. Naray-Szabo, and L. Graf. 1999. The three-dimensional structure of Asp189Ser trypsin provides evidence for an inherent structural plasticity of the protease. Eur. J. Biochem. 263:20-26.
24. Liu, Q., Y. C. Yuan, B. Shen, D. J. Chen, and Y. Chen. 1999. Conformational flexibility of a ubiquitin conjugation enzyme (E2). Biochemistry. 38:1415-1425.
25. Tsou, C. L. 1998. Active site flexibility in enzyme catalysis. Ann. N. Y. Acad. Sci. 864:1-8.
26. Tsou, C. L. 1998. The role of active site flexibility in enzyme catalysis. Biochemistry (Mosc.). 63:253-258.
27. Hinman, L. M., C. R. Coan, and D. A. Deranleau. 1976. Flexibility in the specificity site of serine proteases. Biochemistry. 15:2212-2219.
28. Miller, D. W., and D. A. Agard. 1999. Enzyme specificity under dynamic control: a normal mode analysis of alpha-lytic protease. J. Mol. Biol. 286:267-278.
29. Peters, G. H., and R. P. Bywater, 2002. Essential motions in a fungal lipase with bound substrate, covalently attached inhibitor and product. J. Mol. Recognit. 15:393-404.
30. Kim, S. H., S. V. L. Narayana, and J. E. Volanakis. 1995. Catalytic role of a surface loop of the complement serine-protease factor-D. J. Immunol. 154:6073-6079.
31. DiBella, E. E., and H. A. Scheraga. 1996. The role of the insertion loop around tryptophan 148 in the activity of thrombin. Biochemistry. 35: 4427-4433.
32. Greenwald, J., V. Le, S. L. Butler, F. D. Bushman, and S. Choe. 1999. The mobility of an HIV-1 integrase active site loop is correlated with catalytic activity. Biochemistry. 38:8892-8898.
33. Yang, J., T. Niu. A. Zhang, A. K. Mishra, Z. J. Zhao, and G. W. Zhou. 2001. Relation between the flexibility of the WPD loop and the activity of the catalytic domain of protein tyrosine phosphatase SHP-1. J. Cell. Biochem. 84:47-55.
34. Low, J. C., and S. C. Tu. 2002. Functional roles of conserved residues in the unstructured loop of Vibrio harveyi bacterial luciferase. Biochemistry. 41:1724-1731.
35. Zgiby, S., A. R. Plater. M. A. Bates, G. J. Thomson, and A. Berry. 2002. A functional role for a flexible loop containing Glu 182 in the class II fructose-1,6-bisphosphate aldolase from Escherichia coli. J. Mol. Biol. 315:131-140.
36. Haliloglu, T., I. Bahar, and B. Emian. 1997. Gaussian dynamics of folded proteins. Phys. Rev. Lett. 79:3090-3093.
37. Schellenberger, V., C. W. Turck, and W. J. Rutter. 1994. Role of the S' subsites in serine protease catalysis. Active-site mapping of rat chymotrypsin, rat trypsin, alpha-lytic protease, and cercarial protease from Schistosoma mansoni. Biochemistry. 33:4251-4257.
38. Bahar, I., and R. L. Jemigan. 1997. Inter-residue potentials in globular proteins and the dominance of highly specific hydrophilic interactions at close separation. J. Mol. Biol. 266:195-214.
39. Miyazawa, S., and R. L. Jernigan. 1996. Residue-residue potentials with a favorable contact pair tenu and an unfavorable high packing density term, for simulation and threading. J. Mol. Biol. 256:623-644.
40. Bahar, I., A. R. Atilgan, and B. Erman. 1997. Direct evaluation of thermal fluctuations in proteins using a single-parameter harmonic potential. Fold. Dev. 2:173-181.
41. Haliloglu, T., and I. Bahar. 1999. Structure-based analysis of protein dynamics: comparison of theoretical results for hen lysozyme with X-ray diffraction and NMR relaxation data. Proteins. 37:654-667.
42. Demirel, M. C., A. R. Atilgan, R. L. Jernigan, B. Erman, and I. Bahar. 1998. Identification of kinetically hot residues in proteins. Protein Sci. 7:2522-2532.
43. Wang, Y., A. J. Rader, I. Bahar, and R. L. Jernigan. 2004. Global ribosome motions revealed with elastic network model. J. Struct. Biol. 147:302-314.
44. Keskin, O., S. R. Durell, I. Bahar, R. L. Jernigan, and D. G. Covell. 2002. Relating molecular flexibility to function: a case study of tubulin. Biophys. J. 83:663-680.
45. Kloczkowski, A., J. Mark, and B. Erman. 1989. Chain dimensions and fluctuations in random elastomeric networks. 1. Phantom Gaussian network in the undeformed state. Macromolecules. 22:1423-1432.
46. Flory, P. 1976. Statistical thermodynamics of random networks. Proc. R. Soc. Lond. A. 351:351-380.
47. Wagner, U. G., N. Muller, W. Schmitzberger, H. Falk, and C. Kratky. 1995. Structure determination of the biliverdin apomyoglobin complex: crystal structure analysis of two crystal forms at 1.4 and 1.5 A resolution. J. Mol. Biol. 247:326-337.
48. Micheletti, C., P. Carloni, and A. Maritan. 2004. Accurate and efficient description of protein vibrational dynamics: comparing molecular dynamics and Gaussian models. Proteins. 55:635-645.
49. Bahar, I., B. Erman, R. L. Jernigan, A. R. Atilgan, and D. G. Covell. 1999. Collective motions in HIV-1 reverse transcriptase: examination of flexibility and enzyme function. J. Mol. Biol. 285:1023-1037.
50. Keskin, O., R. L. Jernigan, and I. Bahar. 2000. Proteins with similar architecture exhibit similar large-scale dynamic behavior. Biophys. J. 78:2093-2106.
51. Doruker, P., A. R. Atilgan, and I. Bahar. 2000. Dynamics of proteins predicted by molecular dynamics simulations and analytical approaches: application to alpha-amylase inhibitor. Proteins. 40: 512-524.
52. Xu, C. Y., D. Tobi, and I. Bahar. 2003. Allosteric changes in protein structure computed by a simple mechanical model: hemoglobin T [Lef-right arrow] R2 transition. J. Mol. Biol. 333:153-168.
53. Leitner, T., D. Escanilla, C. Franzen, M. Uhlen, and J. Albert. 1996. Accurate reconstruction of a known HIV-1 transmission history by phylogenetic tree analysis. Proc. Natl. Acad. Sci. USA. 93:10864-10869.
54. Mazurok, N. A., N. V. Rubtsova, A. A. Isaenko, M. E. Pavlova, S. Y. Slobodyanyuk, T. B. Nesterova, and S. M. Zakian. 2001. Comparative chromosome and mitochondrial DNA analyses and phylogenetic relationships within common voles (Microtus, Arvicolidae). Chromosome Res. 9:107-120.
55. Bahar, I., A. R. Atilgan, M. C. Demirel, and B. Emian. 1998. Vibrational dynamics of folded proteins: significance of slow and fast motions in relation to function and stability. Phys, Rev. Lett. 80: 2733-2736.
56. Tsukada, H., and D. M. Blow. 1985. Structure of alpha-chymotrypsin refined at 1.68 Å resolution. J. Mol. Biol. 184:703-711.
57. Emberly, E. G., R. Mukhopadhyay, N. S. Wingreen, and C. Tang. 2003. Flexibility of alpha-helices: results of a statistical analysis of database protein structures. J. Mol. Biol. 327:229-237.
58. Huber, R., D. Kukla, W. Bode, P. Schwager, K. Bartels, J. Deisenhofer, and W. Steigemann. 1974. Structure of the complex formed by bovine trypsin and bovine pancreatic trypsin inhibitor. II. Crystallographic refinement at 1.9 Å resolution. J. Mol. Biol. 89: 73-101.
59. Jernigan, R. L., M. C. Demirel, and I. Bahar. 1999. Relating structure to function through the dominant slow modes of motion of DNA topoisomerase II. Int. J. Quantum Chem. 75:301-312.
60. Jaaskelainen, S., C. S. Verma, R. E. Hubbard, P. Linko, and L. S. Caves. 1998. Confomiational change in the activation of lipase: an analysis in terms of low-frequency normal modes. Protein Sci. 7: 1359-1367.
61. Isin, B., P. Doruker, and I. Bahar. 2002. Functional motions of influenza virus hemagglutinin: a structure-based analytical approach. Biophys. J. 82:569-581.
62. Dauber-Osguthorpe, P., D. J. Osguthorpe, P. S. Stern, and J. Moult. 1999. Low frequency motion in proteins: comparison of normal mode and molecular dynamics of streptomyces griseus protease A. J. Comput. Phys. 151:169-189.
63. Helland, R., H. Czapinska, I. Leiros, M. Olufsen, J. Otlewski, and A. O. Smalas. 2003. Structural consequences of accommodation of four non-cognate amino acid residues in the S1 pocket of bovine trypsin and chymotrypsin. J. Mol. Biol. 333:845-861.
64. Appel, R. D., A. Bairoch, and D. F. Hochstrasser. 1994. A new generation of information retrieval tools for biologists: the example of the ExPASy WWW server. Trends Biochem. Sci. 19:258-260.
65. Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_C windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882.
66. Wroblowski, B., J. F. Diaz, J. Schlitter, and Y. Engelborghs. 1997. Modelling pathways of alpha-chymotrypsin activation and deactivation. Protein Eng. 10:1163-1174.
67. Koradi, R., M. Billeter, and K. Wuthrich. 1996. MOLMOL: a program for display and analysis of macromolecular structures. J. Mol. Graph. 14:51-55.
Wenzhe Ma,*[dagger] Chao Tang,*[double dagger] and Luhua Lai*[dagger]
*Center for Theoretical Biology, and [dagger] State Key Laboratory for Structural Chemistry of Stable and Unstable Species, College of Chemistry, Peking University, Beijing 100871, China; and [double dagger] California Institute for Quantitative Biomedical Research, Departments of Biopharmaceutical Sciences and Biochemistry and Biophysics, University of California, San Francisco, California 94143-2540
Submitted December 6, 2004, and accepted for publication April 21, 2005.
Address reprint requests to Luhua Lai, E-mail: lhlai@pku.edu.cn.
© 2005 by the Biophysical Society
0006-3495/05/08/1183/11 $2.00
Copyright Biophysical Society Aug 2005
Provided by ProQuest Information and Learning Company. All rights Reserved