Abstract
Telithromycin (Ketek[TM], Aventis) is a semisynthetic antibacterial agent belonging to a class of drugs called ketolides, which are a variation on the existing class of antibiotics known as macrolides (e.g., erythromycin), whose structure includes a 14-molecule ring. The FDA approved telithromycin for use as a treatment for upper respiratory tract infections in April of 2004. Its primary use is to treat community-acquired pneumonia and sinusitis. Telithromycin fulfills a role that has arisen due to the rise of microbial resistance to existing macrolides and appears to be effective against macrolide-resistant Streptococcus pneumoniae. The defining differentiating characteristic of the ketolides as opposed to other macrolides is the removal of the neutral sugar, L-cladinose from the 3 position of the macrolide ring and the subsequent oxidation of the 3-hydroxyl to a 3-keto functional group. Telithromycin seems to be an effective antibiotic in the treatment of a variety of skin infections, although double-blind trials have not proven this and currently no indication for treatment of skin infection is being sought from the FDA. Telithromycin also has excellent penetration into the female genial tract and could be useful for treating infections in this area.
**********
Discussion
Telithromycin (Ketek[TM], Aventis) is a semisynthetic antibacterial agent belonging to a class of drugs called the ketolides, a variation on the existing class of macrolides. Telithromycin primary indication is to treat community acquired pneumonia and sinusitis. It is currently available in Germany, France, Italy, Spain and some other countries. On January 8, 2003. the Anti-Infective Drugs Advisory Committee met to recommend approval of telithromycin to treat community-acquired pneumonia, acute exacerbations of chronic bronchitis, and acute bacterial sinusitis (Table 1) (1). The FDA approved telithromycin for use as a treatment for upper respiratory tract infections in April of 2004 and the medication should be available in the United States by July of 2004. While not currently indicated for the treatment of skin infections, it is important for dermatologists to be aware of telithromycin's qualities because telithromycin will be used widely, and it might have utility in the treatment of cutaneous infections.
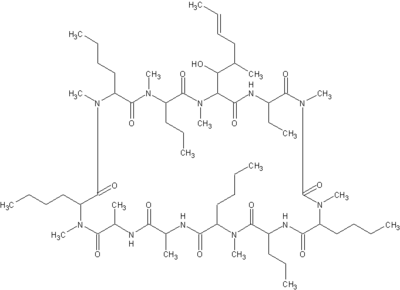
Macrolides are a broad class of antibiotics that include erythromycin (Figure 1), azithromycin (Figure 2), clarthromycin (Figures 3). They share a common ring structure that consists of a 14-member ring of 13 carbon molecules and one oxygen molecule, with various other molecular groups coming off of the ring. The ketolides are semi-synthetic derivatives of the 14-membered macrolide erythromycin A, and retain the erythromycin macrolactone ring structure as well as the D-desosamine sugar attached at position 5. The defining characteristic of the ketolides is the removal of the neutral sugar, L-cladinose from the 3 position of the macrolide ring and the subsequent oxidation of the 3-hydroxyl to a 3-keto functional group (2). Telithromycin (Figure 4) is differentiated from other ketolide compounds by the addition of a large aromatic N-substituted carbamate extension from positions C11/C12 (3).
The structure of ketolides with their ketone group at position 3 of the macrolide ring facilitates intrinsic activity against respiratory tract pathogens and prevents induction of macrolide, lincosamide and streptogramin B resistance. Telithromycin's large aromatic N-substituted C11,12-carbamate side chain also facilitates a more effective interaction with domain II of the bacterial 23S rRNA, enhancing binding to bacterial ribosomes and allowing binding to macrolide, lincosamide and streptogramin B-resistant bacterial ribosomes. Thus ketolides retain activity against macrolide, lincosamide and streptogramin B-resistant bacterial strains (4).
[FIGURE 1 OMITTED]
[FIGURE 2 OMITTED]
[FIGURE 3 OMITTED]
[FIGURE 4 OMITTED]
Telithromycin penetrates rapidly into bronchopulmonary, tonsillar, sinus and middle ear tissues and/or fluids and achieves high concentrations at sites of infection. It also concentrates within polymorphonuclear neutrophils (5). As previously stated, elithromycin inhibits bacterial protein synthesis by action at the 23S rRNA (6). Ketolides have a low potential to select for resistance and cross-resistance both in vitro and in vivo (4). Some infectious disease specialists, however, have questioned whether telithromycin is any more effective against pneumococcal strains resistant to penicillin and/or erythromycin, and suggest that cases of erythromycin cross-resistance have been observed (7). Its dose does not need to be changed in patients with hepatic impairment (8). Its dose, however, should be reduced in patients with renal impairment (9).
Telithromycin fulfills a role that has arisen due to the rise of microbial resistance. Macrolide resistance is increasing with rates estimated to range from 22 to 32% and penicillin resistance rates of 18 to 24% (10). Multi-drug resistance rates are nearing 10%. There is no FDA-approved drug indicated for macrolide-resistance S. pneumoniae. Telithromycin's effect against bacteria compares favorably to azithromycin and clarithomycin (Table 2). Telithromycin appears to be effective against macrolide-resistant S. pneumoniae. Telithromycin is a once-day medication with five to 10 day dosing courses depending on the condition treated (Table 3). At the recommended dosage of 800 mg orally once daily, telithromycin reaches maximal plasma concentrations of about 2 mg/L.
Telithromycin has good activity against gram-negative bacteria and can inhibit production of the toxins that they produce. Shiga toxin-producing Escherichia coli, which plays a crucial role in the stimulation of inflammatory cytokines produced in E. coli, infections have their toxin production inhibited by telithromycin (11).
Telithromycin appears to be a potential useful antibiotic in the treatment of a variety of skin infections. Telithromycin maintains activity against macrolide-resistant pneumococci and Streptococcus pyogenes strains (12). It is a promising treatment for cellulitis because advantages over other macrolides in the treatment of S. pyogenes infection because it is effective against some strains that are resistant to other macrolides (13,14).
Telithromycin has been noted to be effective in the treatment of Staphylococcus aureus as well (15). Telithromycin also has good activity against Borrelia burgdorferi the cause of Lyme disease (16). Telithromycin showed much better activity against corynebacteria (17) and Coxiella burnetii (18) than older macrolides. Double blind clinical trials have yet to be performed for the use of telithromycin to treat skin infection.
Telithromycin has excellent penetration into the female genial tract. It could potentially be a good candidate for the treatment of gynecological infections, including cases associated with sexually transmitted diseases, in particular chancroid (19). In vitro and in vivo antibacterial activities suggest that telithromycin could be a potential candidate for the treatment of bacterial infections complicated by chlamydial infection (20).
Some side effects have been reported with telithromycin. Of these, the most unique side effect is visual disturbances, particularly in slowing the ability to accommodate and the ability to release accommodation. Telithromycin appears to induce diarrhea and nausea as frequently as erythromycin. Rare instances of hepatotoxicity and increased liver enzymes (ALT) are idiosyncratic have been reported with telithromycin (21). Like other macrolides and many newer fluoroquinolones, telithromycin's ability to prolong the QTc interval might be a potential safety issue, especially in elderly patients with predisposing conditions or those who are concurrently receiving drugs that are substrates for CYP2D6 and 3A4. Telithromycin has been associated the development of myasthenia gravis (22,23). The drug interaction profile of telithromycin is similar to that of erythromycin (Table 4). Concomitant administration of it with cisapride or pimozide is contraindicated.
Both azithromycin and clarithromycin have indications against uncomplicated skin and soft-tissue infections, and it would seem that telithromycin should be useful in this regard as well. In conclusion, the ketolide telithromycin is as effective as second-generation macrolides and has similar side-effects and interactions to erythromycin.
References
1. www.fdaadvisorycommittee.com/FDC/AdvisoryCommittee/Committees/Anti- Infective+Drugs/010803_Ketek2/010803_KetekR.htm (accessed June 7, 2003).
2. Zhanel GG, et al. The ketolides: a critical review. Drugs 2002; 62(12):1771-804.
3. Douthwaite S. Structure-activity relationships of ketolides vs. macrolides. Clin Microbiol Infect 2001: 7 Suppl 3:11-7
4. Leclercq R. Will resistance to ketolides develop in Streptococcus pneumoniae? J Infect 2002; 44 Suppl A:11-6.
5. Balfour JA, Figgitt DP. Telithromycin. Drugs 2001; 61:815-29; discussion 830-1.
6. Garza-Ramos G, et al. Binding site of macrolide antibiotics on the ribosome: new resistance mutation identifies a specific interaction of ketolides with rRNA. J Bacteriol 2001; 183:6898-907.
7. Telithromycin: new preparation. A needless addition to the other macrolides. Prescrire Int 2003; 12:8-11.
8. Cantalloube C, et al. Pharmacokinetics of the ketolide telithromycin after single and repeated doses in patients with hepatic impairment. Int J Antimicrob Agents 2003; 22:112-21.
9. Shi J, et al. Pharmacokinetics and safety of the ketolide telithromycin in patients with renal impairment. J Clin Pharmacol 2004; 44:234-44.
10. http://www.liquent.com/Downloads/adcomm_sampleissue.pdf (accessed June 7, 2003).
11. Nakagawa S, et al. Inhibitory action of telithromycin against Shiga toxin and endotoxin. Biochem Biophys Res Commun 2003; 310:1194-9.
12. Mensa J, Garcia-Vazquez E, Vila J. Macrolides, ketolides and streptogramins. Enferm Infecc Microbiol Clin 2003; 21:200-7; quiz 208, 219.
13. Ioannidou S, et al. In vitro activity of telithromycin (HMR 3647) against Greek Streptococcus pyogenes and Streptococcus pneumoniae clinical isolates with different macrolide susceptibilities. Clin Microbiol Infect 2003; 9:704-7.
14. Bozdogan B, et al. Activity of telithromycin compared with seven other agents against 1039 Streptococcus pyogenes pediatric isolates from ten centers in central and eastern Europe. Clin Microbiol Infect 2003; 9:741-5.
15. Jacobs MR, Bajaksouzian S, Appelbaum PC. Telithromycin post-antibiotic and post-antibiotic sub-MIC effects for 10 Gram-positive cocci. J Antimicrob Chemother. 2003; 52:809-12.
16. Hunfeld KP, et al. Comparison of in vitro activities of ketolides, macrolides, and an azalide against the spirochete Borrelia burgdorferi. Antimicrob Agents Chemother. 2004; 48:344-7.
17. Sanchez Hernandez J, et al. In vitro activity of newer antibiotics against Corynebacterium jeikeium, Corynebacterium amycolatum and Corynebacterium urealyticum. Int J Antimicrob Agents 2003; 22:492-6.
18. Boulos A, et al. Measurement of the antibiotic susceptibility of Coxiella burnetii using real time PCR. Int J Antimicrob Agents 2004; 23:169-74.
19. Mikamo H, Ninomiya M. Tamaya T. Penetration of oral telithromycin into female genital tissues. J Infect Chemother 2003; 9:358-60.
20. Mikamo H. et al. In vitro and in vivo antibacterial activities of telithromycin. Chemotherapy 2003; 49:62-5.
21. Shain CS, Amsden GW. Telithromycin: the first of the ketolides. Ann Pharmacother 2002; 36:452-64.
22. Nieman RB, et al. Telithromycin and myasthenia gravis. Clin Infect Dis 2003; 37:1579.
23. Moreno Alvarez PJ, Madurga Sanz M. Telithromycin and exacerbation in myasthenia gravis. Farm Hosp 2003; 27:199-200.
NOAH SCHEINFELD MD
ST. LUKE'S-ROOSEVELT HOSPITAL CENTER AND BETH ISRAEL MEDICAL CENTER
NEW YORK, NEW YORK
ADDRESS FOR CORRESPONDENCE:
Noah Scheinfeld MD
Department of Dermatology
St. Luke's-Roosevelt Hospital Center
1090 Amsterdam Ave, Suite 11 D
New York, NY 10025
Phone: (212) 523-3888
Fax: (212) 523-3808
E-mail: Scheinfeld@earthlink.net
COPYRIGHT 2004 Journal of Drugs in Dermatology, Inc.
COPYRIGHT 2005 Gale Group