Abstract
This is a preliminary report of an ongoing study to test the efficacy of intralesional injections of the antiviral drug cidofovir in adults with recurrent laryngeal papillomas in whom multiple other treatments have previously failed. This study has been designed to include 10 to 20 patients, a number sufficient to either prove or disprove the safety and efficacy of this agent. This report conveys information on the first three patients enrolled in the trial. Each patient received an overall dose of 5 to 10 ml of cidofovir, at a concentration of 4.17 mg/ml, intralesionally at 2- to 4-week intervals. The approximate volume injected into each wart was 0.2 to 0.5 ml. Biopsies of the lesion sites were obtained at the initiation and completion of therapy. No other treatment was given. Resolution of lesions was monitored by videolaryngoscopy and still photography 1 to 2 weeks after each treatment. In time, the lesions resolved in all three patients, although all three later experienced a minor recurrence. We conclud e that intralesional cidofovir appears to be a promising new treatment for controlling--and perhaps at higher dosages curing--refractory laryngeal papillomas, while causing little or no injury to laryngeal structures.
Introduction
Laryngeal papillomas, which were originally described in the 17th century as "warts in the throat," occur in two age groups: adults and young children. There is no distinct difference in the histology of the disease between these two groups of patients. Laryngeal papillomas are notoriously unpredictable in their clinical behavior, and treatment can be extremely frustrating and prolonged. Because of the highly recurrent nature of this disease, most patients require multiple surgical procedures. Laryngeal injury from scarring is common. The disease causes a good deal of psychological stress--and in many instances financial hardship--for patients and their families.
Laryngeal papillomas are caused by an infection with human papillomavirus (HPV). The mechanisms of acquisition and transmission of HPV in patients with laryngeal papillomas have not been determined, nor have the specific etiologic factors that predispose certain adults to HPV. Currently, no medical or surgical cure exists, although palliative interventions (e.g., laser ablation) are routinely employed. Although some cases of laryngeal papilloma regress over time, particularly around adolescence, others become long-term, chronic management problems that last for decades. In such cases, the lesions can undergo a malignant transformation [1,2] or lead to involvement of the tracheopulmonary tree.
The antiviral medication cidofovir was recently approved for the treatment of cytomegalovirus retinitis in patients with acquired immunodeficiency syndrome. The drug's efficacy has also been suggested in the treatment of esophageal papillomas, as reported in one patient by Van Cutsem et al, [3] and in the treatment of laryngeal papillomas in children and adults, as reported by Snoeck et al. [4] Both of these studies were uncontrolled.
The purpose of the phase I/Ia study described in this report is to define the safety and efficacy of intralesional injections of cidofovir in adults with respiratory papillomatosis. Although this drug can be administered intravenously, or possibly in a nebulized form, it appears that the safest and most controlled method of drug delivery is by local injection at the tumor site. This is the route that is being used in this study.
Materials and methods
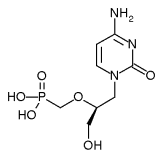
This preliminary report describes the outcomes of the first three patients who entered the study. Following enrollment, each of the three patients underwent endoscopy in the surgical suite. Tracheobronchial and bronchial examinations were performed, and all lesions were treated by injecting cidofovir into each of the papillomas and the surrounding mucosa. Cidofovir (Vistide; Gilead Sciences) is a new compound, 1-[(S)-3-hydroxy-2-phosphonomethoxy)propyl]cytosine dihydrate, that is effective in the destruction of a broad range of DNA viruses. The maximum total volume of drug administered during the endoscopic procedure was determined by the number of lesions and the size of the patient's airway. All three patients received the medication within the bounds of current Food and Drug Administration dosing recommendations (5 mg/kg for intravenous use) and well below any levels that have been reported to lead to toxicity. The concentration of cidofovir was 4.17 mg/ml, and each patient received a total dose of 5 to 10 ml. The approximate volume injected into each wart was 0.2 to 0.5 ml. This amount was determined by direct observation of the amount necessary to blanch each wart. The maximum amount of medication any patient received did not exceed 1 mg/kg.
One to 2 weeks following each administration of cidofovir, patients were evaluated by rigid and flexible laryngoscopy at the George Washington University Voice Treatment Center. Lesions were reinfiltrated with cidofovir in the operating room every 2 to 4 weeks. When papillomas were no longer seen on endoscopy, cidofovir was administered one more time at the site of the lesion, and a biopsy was taken. When lesions were still not visible during two consecutive rigid laryngoscopic office examinations, treatment was suspended and the patients continued to be evaluated by rigid or flexible laryngoscopy every 3 months. When lesions reappeared, cidofovir injections resumed and were administered every 2 weeks, with endoscopic followup, until they were again no longer seen on endoscopy.
All patients were seen for followup at 3, 6, 12, 18, and 24 months after enrollment, regardless of the timing of their cidofovir administration.
Results
Patient #1. In June 1989, a then-26-year-old physician and professional singer noted persistent hoarseness following a recording session. He was subsequently diagnosed with laryngeal papilloma. His papillomas were positive for HPV types 6 and 11. Tumors were present on the false vocal folds, the true folds, and the subglottis. Over the years, this patient underwent 65 excisions with a [CO.sub.2] laser, but the disease did not resolve. Over the next several years, he underwent treatment at various times with interferon alpha-nl, thymosine, isoprinosine, and photodynamic therapy.
Eventually, he was treated with seven sessions of cidofovir intralesional injections over a 6-month period during 1994 and 1995 in Belgium. He did well until May 1998, when a recurrence was noted on the anterior vocal folds. He then came to The George Washington University Medical Center and received two more injections and achieved a complete response. He remained lesion-free until late 1999, when he experienced a minor recurrence, which quickly responded to additional injections.
Patient #2. A 63-year-old aerospace engineer came to us in September 1997 with a 30-plus-year history of recurring laryngeal papillomas. He had undergone at least 65 direct laryngoscopies and sharp and [CO.sub.2] laser excisions. His papillomas were positive for HPV types 6 and 11. Over the years, he required two tracheotomies.
After he entered this study, he received 15 injections of cidofovir. Initially, the injections were administered every 2 to 4 weeks, but the interval was gradually lengthened to every 3 months, Treatment was discontinued when his larynx appeared to be tumor-free on rigid laryngoscopy (figure 1). Three months later, he experienced the simultaneous development of several small areas of recurrence, and intralesional injections of cidofovir were resumed with good results. No other treatment has been necessary.
Patient #3. A 30-year-old executive was referred to us for treatment of laryngeal papillomas. His disease involved both vocal folds and the anterior commissure, which had been unsuccessfully treated with [CO.sub.2] laser ablations. His papillomas were positive for HPV types 6 and 11. Following intralesional cidofovir injections over a 4-month period, the disease appeared to have resolved completely (figure 2). A biopsy was taken of the laryngeal mucosa where the papillomas had been, and it was reported and normal on light microscopy. Six months after his last injection, the man was noted to have a recurrence on one vocal fold. He underwent microlaryngoscopy with injection, and has since experienced an apparent resolution of the remaining lesion.
Discussion
After a series of intralesional cidofovir injections, which were not supplemented by any other therapy, all three patients experienced a complete resolution of their papillomas on visual examination. Eventually, however, all three patients experienced a minor recurrence (patient #1 after 18 months, patient #2 after 3 months, and patient #3 after 6 months). All these recurrences responded to further intralesional injections.
Thus far, the treatment of the three adults in our study has been effective, but as yet not curative. The concentration of cidofovir was relatively modest (4.17 mg/ml). As our study progresses, we will increase the concentration to 6.25 mg/ml, which we hope will lead to a more permanent response.
There is some concern regarding cidofovir's potential carcinogenic effect. In the original group studied by Snoeck et al, three of 17 patients had squamous cell carcinoma in situ, but two of them had already developed it prior to treatment. [4] All three were smokers.
The advantages of intralesional cidofovir treatment are important. First, unlike other treatments, the injections do not appear to injure the larynx. Therefore, even anterior commissure lesions can be injected without fear of causing web formation. Second, injections are safer than laser therapy, which carries a risk of fire and a risk of the virus spreading from the laser's vapor plume to the patient's lower tracheobronchial tree or to operating room personnel. Third, injection therapy requires far less time under anesthesia than does excision. Finally, patients report that they experience less postoperative discomfort.
It appears that intralesional injections of cidofovir have the potential to be an important adjunct to the treatment of laryngeal papilloma. With further refinement of the dosage and frequency schedules, it could in time become a principal therapy for this disease.
From the Division of Otolaryngology-Head and Neck Surgery, The George Washington University Medical Center, Washington, DC. The information in this article was first presented at the Southern Section Meeting of the Triological Society in New Orleans on Jan. 16, 1999.
References
(1.) Andrews TM, Myer CM. Malignant (atypical) carcinoid of the larynx occurring in a patient with laryngotracheal papillomatosis. Am J Otolaryngol 1992;13:238-42.
(2.) Doyle DJ, Henderson LA, LeJeune FE Jr., Miller RH. Changes in human papillomavirus typing of recurrent respiratory papillomatosis progressing to malignant neoplasm. Arch Otolaryngol Head Neck Surg 1994;120:1273-6.
(3.) Van Cutsem E, Snoeck R, Van Ranst M, et al. Successful treatment of a squamous papilloma of the hypopharynx-esophagus by local injections of (S)-l-(3-hydroxy-2-phosphonylmethoxypropyl) cytosine. J Med Virol 1995;45:230-5.
(4.) Snoeck R, Wellens W, Desloovere C, et al. Treatment of severe laryngeal papillomatosis with intralesional injections of cidofovir [(5)-1-(3-hydroxy-2-phosphonylmethoxypropyl) cytosine]. J Med Virol 1998;54:219-25.
COPYRIGHT 2000 Medquest Communications, Inc.
COPYRIGHT 2000 Gale Group