Note: AIDS Treatment News published this article to provide background for activists who might want to help support the political will to make this long-delayed, lifesaving treatment available to those who need it.
On November 22, 2004, days alter The Lancet reported that the cheap antibiotic co-trimoxazole (Septra, Bactrim, and other brand names) had dramatically reduced death in a group of Zambian children with HIV, the World Health Organization (WHO), UNAIDS and UNICEF released a statement recommending the drug for all children with HIV symptoms in poor countries [1]. But activists say the global health authorities' seemingly quick action came years--even decades--late, and it will take a lot more work to actually deliver the drug's lifesaving promise.
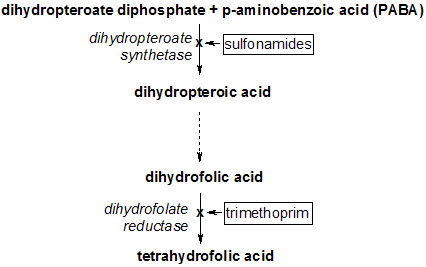
Co-trimoxazole (a combination of trimethoprim and sulfamethoxazole, sometimes called TMP/SMX) was first used to prevent AIDS-related PCP (pneumocystis pneumonia) in 1985 [2]--(although it was standard of care for prevention of PCP in other patients with immune deficiencies long before then). It also prevents toxoplasmosis in people with AIDS [3]. Between 1987 and 1992 (before combination antiretroviral treatment), the drug cut U.S. deaths from PCP by more than half [4]. Yet today, in African countries where very few can get antiretrovirals, co-trimoxazole is still hard to come by, despite its low cost.
"We do not have good estimates of how many children are getting cotrim," says the WHO's Dr. Siobhan Crowley, "but our sense from the field is that it is not enough."
Brook Baker, a Northeastern University law professor and activist with the Health GAP (Global Access Project), says children are particularly neglected, in everything from prevention to prophylaxis to antiretroviral therapy. "Mother-to-child transmission prevention reaches only 10 percent of pregnant women in Africa at best," he says. "Follow-up for children with antiretrovirals, prophylaxis, or OI medicines is a total mess. Fifty percent of HIV-positive kids die before age 2, and yet drug companies are not investigating pediatric interventions and pediatric formulations."
Previously, WHO recommended co-trimoxazole for all newborns of women with HIV and to children with low CD4 counts or an AIDS diagnosis [5]. Worried about heavy resistance to the drug in some parts of Africa (where it is used to treat other infections like dysentery and malaria) WHO did not suggest more widespread distribution--such as to children with some symptoms but no access to HIV testing--until now. "Concerns that this would not be effective in areas of high resistance ... do not seem to have been shown to be real given this study data," Crowley says.
Dr. Diana Gibb of the UK's Medical Research Council and her British and Zambian colleagues conducted the November Lancet study in an area with high bacterial resistance to the medicine. Of children taking co-trimoxazole, 28 percent died, while 42 percent who took placebo died. No allergic reactions occurred. Researchers stopped the trial early so that all children enrolled could get the successful drug [6].
For Baker, the delay in carrying out this research was more than tragic. "Twenty years into the plague, we're now looking closely at prophylaxis to protect kids," he says. "It's outrageous."
But the wait for widespread access may have just begun. Since 2001, WHO has recommended cotrimoxazole for adults with symptomatic HIV disease or below 500 CD4s, and pregnant women [5]. But the authors of an October Lancet study of co-trimoxazole in Uganda report that the drug is still "rarely used in Africa"--and that Uganda is only now, because of that study, developing a co-trimoxizole policy [6].
Crowley says WHO will "strongly advocate for greater coverage of prophylactic cotrim for both adults and children," and "ensure governments hear about it." The drug is cheap--only $10 a year per child--and WHO advises countries to distribute it free [1, 8]. But they will also need training for health care providers, not to mention the desperate scarcity of clinics themselves. David Hoos, MD, who conducts international HIV training, technical assistance and drug procurement programs for Columbia's Mailman School of Public Health, says that lack of access to antiretrovirals has meant that Africans with HIV aren't even being brought into regular care where they could get prophylaxis for opportunistic infections.
"People need to come in every month for continuity of care," he says. "It wasn't historically a question of [cotrimoxazole] being expensive--the facilities are much more expensive." Hoos hopes major projects like the Global Fund to Fight AIDS, Tuberculosis and Malaria, and the President's Emergency Plan for AIDS Relief (PEPFAR) will bolster Africa's health care infrastructure.
UNICEF's Liza Barrie says her organization will work with WHO and other partners this year to support the following: adaptation of co-trimoxazole treatment guidelines; training; development of new tools to forecast the number of children who will need the drug; and procurement services through UNICEF Supply Division. But it looks like the degree of involvement international agencies pursue might depend on the kind of pressure that is put on them to take action. WHO, UNAIDS and UNICEF, for their part, urge others to act. "Greater advocacy liar the use of co-trimoxazole prophylaxis in children is urgently required," their statement reads [1].
References
[1.] Joint WHO/UNAIDS/UNICEF statement on use of cotrimoxazole as prophylaxis in HIV exposed and HIV infected children, November 2004, http://www.unaids.org/EN/media/press+releases.asp (This site is awkward to use, but try the "Simple search" for "prophylaxis" WITHOUT the quotation marks, re-sort by date if necessary, and look down the list of titles returned. After clicking on the title, click on "Download the full PDF version" to get all four pages of the document. If the search finds nothing and your spelling is correct, try a different Web browser.)
[2.] Pneumocystis pneumonia (PCP) fact sheet, New Mexico AIDS InfoNet, http://www.aidsinfonet.org/articles.php?articleID=515&newLang=en
[3.] Can toxoplasmosis be prevented?, http://www.aidsmeds.com/OIs/Toxo4.htm
[4.] Pneumocystis carinii pneumonia (PCP), by Michael Marco, http://www.aidsinfonyc.org/tag/comp/ois98/16.html
[5.] Provisional WHO/UNAIDS secretariat recommendations on the use of cotrimoxazole prophylaxis in adults and children living with HIV/AIDS in Africa, March 2000, http://www.unaids.org and search on Cotrimoxazole. Or: http://www.unaids.org/NetTools/Misc/DocInfo.aspx?LANG =en&href=http%3a%21%2fgva-doc-owl%2fWEBcontent%2fDocuments%2fpub%2fPublication s%2fIRC-pub04%2frecommendation._en%26%2346%3bpdf (then click "Download the full PDF version").
[6.] C Chintu, GJ Bhat, AS Walker, and others. Cotrimoxazole as prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): a double-blind randomized placebo-controlled trial. Lancet 2004; volume 364, pages 1865-71.
[7.] J Mermin, J Lule, JP Ekwaru and others. Effect of cotrimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. Lancet 2004; volume 364: pages 1428-34.
[8.] MDs urge antibiotics for children with HIV, The Star Ledger, New Jersey, November 19 2004.
COPYRIGHT 2004 John S. James
COPYRIGHT 2005 Gale Group