More than 4 percent of preschool-aged children in the United States have blood lead levels above 10 [micro]g per dL (0.50 [micro]mol per L), and these levels have been associated with a decline in IQ. The Centers for Disease Control and Prevention advocates the use of a screening questionnaire to identify lead exposure or toxicity in all children. Primary prevention through the removal of lead from gasoline and paint has led to a reduction of blood lead levels in children. Secondary prevention through paint hazard remediation is effective in homes that have a high lead burden. Children with lead levels of 45 to 69 [micro]g per dL (2.15 to 3.35 [micro]mol per L) should receive chelation therapy using succimer (DMSA) or edetate calcium disodium (CaNa2EDTA). Use of both CaNa2EDTA and dimercaprol (BAL in oil) is indicated in children with blood lead levels higher than 70 [micro]g per dL (3.40 [micro]mol per L). Current treatment recommendations are based on the reduction of blood lead levels, which may not represent a significant overall reduction of the lead burden. Clinical trials of existing agents are needed to determine patient-oriented outcomes, such as the effect on IQ. (Am Fam Physician 2000;62:545-54,559-60.)
Lead poisoning has been a significant public health problem for centuries. Studies conducted in the United States since 1950 have shown the adverse effects of even low levels of lead exposure. Consequently, lead poisoning is now defined as a blood lead level equal to or greater than 10 [micro]g per dL (0.50 [micro]mol per L).(1)
In the United States, about 4.4 percent of children between one and five years of age have blood lead levels above 10 [micro]g per dL.(2) Elevated levels of lead are found significantly more often in black children, children from low-income families and children who live in urban areas.(2) In 1995, 14 million children younger than eight years lived in housing that contained high concentrations of lead paint.(1) Other common sources of exposure include lead-soldered pipes, lead in soil, lead in dust from home-remodeling projects, lead chromate in coated electrical wire, lead-glazed ceramics, leaded crystal and lead-soldered cans manufactured in foreign countries.(3)
Adverse Health Effects of Lead Poisoning
Blood lead levels below 70 [micro]g per dL (3.40 [micro]mol per L) can result in damage to the central nervous system, kidneys and hematopoietic system. Lead toxicity is associated with a two- to three-point decrease in IQ test scores for every increase of 10 [micro]g per dL in the blood lead level.(1,4) Elevated blood lead levels are also associated with neurodevelopmental abnormalities, including attention deficit disorder, behavioral disturbances, learning disabilities and deficits in fine and gross motor development.(4-7)
Toxic effects on the central nervous system and resultant long-term neurobehavioral and cognitive deficits occur even with mildly elevated blood lead levels (10 to 25 [micro]g per dL [0.50 to 1.20 [micro]mol per L]).(6-8) At high blood lead levels (more than 70 [micro]g per dL), nephropathy, neuropathy, increased intracranial pressure, seizures and death are common.(3,5)
Lead toxicity is also associated with renal defects in vitamin D metabolism and microcytic anemia.(3,9)
Screening Protocols
The Centers for Disease Control and Prevention (CDC) and the American Academy of Pediatrics (AAP) have addressed the societal burden of lead poisoning10 by issuing recommendations for childhood lead screening.(4,5) These recommendations include universal screening for lead poisoning in areas where at least 27 percent of houses were built before 1950 and in populations in which 12 percent or more of one- and two-year-old children have elevated blood lead levels. In all other areas, the CDC promotes targeted blood lead screening of children between six months and six years of age based on positive responses to one or more items on a screening questionnaire(3,4,11,12) (Table 1).(5)
The American Academy of Family Physicians recommends lead screening at 12 months of age in infants who meet the following criteria(13):
1. Residence in a community with a high or undefined prevalence of lead levels requiring intervention.
2. Residence in or frequent visits to a home built before 1950 that has dilapidated paint or has recently undergone or is undergoing renovation or remodeling.
3. Close contact with a person who has an elevated blood lead level.
4. Residence near a lead industry or heavy traffic.
5. Residence with a person whose hobby or job involves lead exposure.
6. Use of lead-based pottery.
7. Use of traditional remedies that contain lead.
Universal blood lead screening is somewhat controversial.(14) Physicians should check with the local health department to determine if universal screening is necessary in that geographic area. When possible, venous blood samples should be used for initial screening. If capillary (fingerstick) blood testing is used initially, values above 10 [micro]g per dL should be confirmed with a venous sample (Table 2).(4)
Prevention Strategies primary prevention
Strategies for the primary prevention of lead poisoning have only been implemented within the past 25 years. One such measure, the Environmental Protection Agency's mandated removal of lead from gasoline, resulted in a 73 percent decrease in gasoline-related lead consumption between 1975 and 1984. This action coincided with a 37 percent reduction in blood lead levels from 1976 through 1980. A second major primary prevention strategy, the removal of lead from paint, was mandated in 1978. Cost benefits for the removal of lead from gasoline and paint have been reported.(15)
Additional primary prevention strategies have been proposed by the U.S. Department of Health and Human Services and have been endorsed by the CDC.(16)
SECONDARY PREVENTION
Secondary prevention of lead poisoning includes family education, high-efficiency particulate air (HEPA) vacuums, interior dust abatement, soil abatement and residential paint hazard remediation. The efficacies of environmental lead removal techniques are reviewed in Table 3.
In one study,(17) researchers visited families and provided literature on lead poisoning, information on behaviors that increase the potential for lead exposure, instructions on proper nail care and diet for children, and suggestions for the removal of peeling paint. These interventions were associated with a 40 percent decline in blood lead levels over one year in children who had an average initial blood lead level of 15 [micro]g per dL (0.70 [micro]mol per L). Because the study did not report the frequency of paint removal in these homes, the effect of education as a sole intervention is uncertain.
HEPA vacuums retain lead-containing particles that slip through the filters of conventional vacuum cleaners.(18) Dust control strategies include the use of an all-purpose cleaner or detergent solution to wipe or mop the floors, walls and horizontal surfaces of lead-contaminated dwellings. Most studies have not found HEPA vacuuming and dust control measures to be efficacious,(18,19) although one recent randomized, controlled trial(20) of these methods demonstrated a 34 percent reduction in the blood lead levels of children whose homes were treated 20 or more times during a one-year period.
Soil abatement is accomplished by removing the top 15 cm (6 in) of soil from the yard, covering the exposed subsurface with geotextile fabric and then replacing the top layer with 20 cm (8 in) of clean soil and ground cover.(3) This intervention has been associated with a decline in blood lead levels of only 0.8 to 2.7 [micro]g per dL (0.05 to 0.15 [micro]mol per L) in children with low to moderate baseline lead levels.(21,22) Based on study findings, soil abatement is an expensive and low-yield intervention in most children with low-level lead exposure.
Paint hazard remediation entails removing lead paint from surfaces 1.5 m (5 ft) or less above the ground or covering lead paint on these surfaces, and removing loose paint from other surfaces in the living area.(21) This intervention has been associated with an 18 to 23 percent decline in the blood lead levels of children with baseline lead levels of 25 [micro]g per dL or higher.(23,24) The greatest effect of paint hazard remediation is seen in children with preabatement blood lead levels of 35 [micro]g per dL (1.70 [micro]mol per L) or higher.(24)
Paint hazard remediation in homes having a low lead burden has not been found to result in a clinically significant decline in blood lead levels. In fact, the intervention may actually increase blood lead levels.(21,23)
New statutes have curtailed abatement techniques thought to be associated with transient increases in residents' blood lead levels during remediation. These techniques include dry abrasive blasting and on-site use of methylene chloride or propane torches.(25)
Pharmacologic Treatment
If a child is found to have a significantly elevated blood lead level (greater than 45 [micro]g per dL [2.15 [micro]mol per L]), chelation therapy should be considered (Tables 2(4) and 4(26)). Specific treatment recommendations are based on the measured blood lead level. Available chelation agents include the following:
1. Dimercaprol, or BAL (British antilewisite) in oil.
2. Edetate disodium calcium, or CaNa2EDTA (calcium disodium salt of ethylenediaminetetraacetic acid [EDTA]).
3. Succimer, or DMSA (2,3-dimercaptosuccinic acid).
4. Penicillamine (Cuprimine). Note that the U.S. Food and Drug Administration (FDA) has not labeled penicillamine for the treatment of lead poisoning.
The efficacies of pharmacologic treatments for lead poisoning are reviewed in Table 5. dimercaprol (bal) Dimercaprol, or BAL, binds with lead and is excreted in bile and urine. In children with blood lead levels exceeding 70 [micro]g per dL, BAL is used in combination with CaNa2EDTA to chelate lead in the brain. BAL is mixed in peanut oil. The dosage is 25 mg per kg per day given intramuscularly in six divided doses (4.16 mg per kg per injection) for two to five days.(16,26)
Common side effects of BAL include mild febrile reactions, mild elevation of liver transaminase levels, nausea and vomiting, headache, conjunctivitis, lacrimation, rhinorrhea and salivation.(16) To avoid a possible toxic reaction, iron supplementation for lead-caused anemia should be deferred until BAL therapy has been completed.(26)
BAL treatment is contraindicated in patients with peanut allergies. This chelation agent can cause hemolysis in patients with glucose-6-phosphatase deficiency.(16)
EDETATE DISODIUM CALCIUM (CANA2EDTA)
Edetate disodium calcium, or CaNa2EDTA, binds with extracellular lead and increases urinary lead excretion. This chelation agent should not be confused with disodium edetate (NaEDTA), which can cause fatal hypocalcemia.(26)
CaNa2EDTA is recommended as monotherapy for the treatment of children with blood lead levels between 45 and 69 [micro]g per dL (2.15 and 3.35 [micro]mol per L). It is also recommended for use in combination with BAL for the treatment of blood lead levels higher than 70 [micro]g per dL.(16,26)
The appropriate dosage of CaNa2EDTA is 25 to 50 mg per kg per day for five days, depending on the initial blood lead level.(16,26) This agent is usually given intravenously in a solution of less than 0.5 percent dextrose and water or in a 0.9 percent saline solution. Slow infusion of CaNa2EDTA over four hours is indicated because rapid infusion may precipitate encephalopathy. Intramuscular administration is possible but causes extreme pain if CaNa2EDTA is not mixed with procaine.
Common side effects of CaNa2EDTA therapy include proteinuria, urinary sedimentation and transient elevations of blood urea nitrogen, creatinine and liver enzyme levels. Adequate hydration is essential when CaNa2EDTA is used, and patients should be monitored closely for signs of renal toxicity and oliguria.(16)
The findings of several studies support the use of CaNa2EDTA in children with blood lead levels above 45 [micro]g per dL.(27,28) Perhaps the most important of these investigations is a prospective study in 154 children who had initial blood lead levels of 25 to 55 [micro]g per dL (1.20 to 2.65 [micro]mol per L).(27) This study compared the use of CaNa2EDTA therapy plus home lead abatement with the sole use of home lead abatement. The investigators found that the children treated with CaNa2EDTA had a one-point increase in IQ test scores over six months for every 3 [micro]g per dL (0.15 [micro]mol per L) decrease in blood lead levels. Whether such a minimal increase in IQ test scores has any functional significance remains to be seen.
SUCCIMER (DMSA)
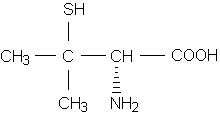
Succimer, or DMSA, is the only available oral agent for lead chelation. This agent is approved for use in children with blood lead levels of 45 to 69 [micro]g per dL. The recommended dosage is 10 mg per kg taken every eight hours for five days, then every 12 hours for two weeks. Multiple courses may be given, with a minimum of two weeks between courses.(16,26)
Drawbacks of succimer therapy include a "rotten egg" sulfur odor, abdominal pain, nausea and vomiting, diarrhea, and possible elevation of hepatic transaminase levels.(3) Like any chelation agent, DMSA should be given with aggressive home lead abatement to avoid continued lead toxicity.(16)
Because DMSA therapy has been associated with 43 to 60 percent reductions in blood lead levels,(29,30) the outlook is favorable for its use in children with low-level lead poisoning. DMSA has fared well in studies comparing it to other chelation agents.
One retrospective study(31) that compared children treated with BAL and CaNa2EDTA and children treated with DMSA and CaNa2EDTA found a comparable reduction in post-treatment blood lead levels in the two groups but fewer adverse treatment effects in the DMSA group. In two small, noncontrolled clinical trials(32,33) comparing the use of DMSA and CaNa2EDTA in children with initial blood lead levels of 31 to 69 [micro]g per dL (1.50 to 3.35 [micro]mol per L), high-dose DMSA therapy reduced mean blood lead levels by 61 to 77 percent over five days. In both studies, the blood lead levels of the children treated with CaNa2EDTA declined by about 45 percent.(32,33)
In contrast, a recently published randomized, controlled trial of DMSA versus placebo in conjunction with environmental remediation in children who had baseline blood lead levels of 30 to 45 [micro]g per dL (1.45 to 2.15 [micro]mol per L) found similar reductions in lead levels after six months.(34) However, this study of 39 children may have lacked the power to detect a statistical difference between the DMSA and placebo groups.
Based on research and experience, the CDC and AAP recommend succimer therapy for children with blood lead levels above 45 [micro]g per dL. Although some health centers use DMSA in children with lower levels of lead poisoning, the scientific literature demonstrating the efficacy of this agent at lower levels is inconclusive.
We believe that succimer should not be given routinely to children with blood lead levels below 45 [micro]g per dL, although there may be a place for its use on a case-by-case basis in coordination with a pediatric specialist. A large randomized, controlled trial is currently in progress to assess the efficacy of DMSA in children with moderately elevated lead levels (20 to 44 [micro]g per dL [0.95 to 2.10 [micro]mol per L]).(35) The findings of the study may lend support to the use of succimer in children with lower blood lead levels.
PENICILLAMINE
Although the FDA has not labeled penicillamine for the treatment of lead poisoning, one retrospective study(36) found that this agent reduced blood lead levels by 33 percent in children with initial levels of 25 to 40 [micro]g per dL (1.20 to 1.95 [micro]mol per L).
Adverse effects of penicillamine include rash, leukopenia, thrombocytopenia, hematuria, proteinuria, eosinophilia, elevated liver enzyme levels and nephrotoxicity. Because of the toxicity of penicillamine, the AAP recommends that this agent be used to treat lead poisoning "only when unacceptable reactions have occurred to DMSA and CaNa2EDTA and continued therapy is considered important."(26(p158))
If penicillamine is used, the appropriate dosage is 20 to 30 mg per kg per day in divided doses taken for up to several months. Children should be started on a small dose that is gradually increased. Complete blood count and urinalysis should be monitored routinely throughout treatment. Penicillamine therapy is contraindicated in children with known penicillin allergy.(16)
Management of Lead Toxicity
Children with elevated blood lead levels should undergo clinical evaluation (Table 6).(5) The proper management of children with blood lead levels higher than 10 [micro]g per dL is based on the relative degree of the elevation. The CDC recommends a coordinated program of follow-up screening, education, case management, environmental investigation, lead hazard control and clinical evaluation.(16) The AAP recommendations, summarized in Tables 24 and 4,(26) differ only slightly from the CDC recommendations.
BLOOD LEAD LEVELS OF 10 TO 19 [MICRO]G PER DL
For children with blood lead levels between 10 and 19 [micro]g per dL (0.5 to 0.9 [micro]mol per L), the CDC recommends nonpharmacologic interventions. Family education should include information on sources of lead exposure, potential adverse health effects, good nutrition and control of lead dust.
BLOOD LEAD LEVELS OF 20 TO 44 [MICRO]G PER DL
For children with blood lead levels between 20 to 44 [micro]g per dL, strategies recommended by the CDC include case management by a qualified social worker, clinical management, environmental assessment and lead hazard control.(5) Local health departments are valuable resources for providing lead education, coordinating home inspections and helping with financial assistance or home relocation. Appropriate medical management involves a thorough clinical evaluation and possible cautious use of chelation therapy in children with refractory blood lead levels.
BLOOD LEAD LEVELS OF 45 TO 69 [MICRO]G PER DL
The CDC and AAP support the use of chelation therapy in children with blood lead levels between 45 and 69 [micro]g per dL. The CDC recommends CaNa2EDTA monotherapy.(16) The recommended dosage is 25 mg per kg per day given by continuous infusion or in divided doses for no more than five days, with regular monitoring of blood electrolyte levels and renal and hepatic function during treatment.(26) A second course of CaNa2EDTA may be given if the blood lead level rebounds to 45 [micro]g per dL within seven to 14 days after treatment.
In children without encephalopathy, the AAP supports the use of DMSA for the treatment of blood lead levels of 45 to 69 [micro]g per dL. The recommended dosage is 30 mg per kg per day for five days, followed by 20 mg per kg per day for 14 days.(26)
Hospitalization may be warranted initially to monitor side effects and implement proper environmental lead abatement.(26) blood lead levels greater than 70 [micro]g per dl Children with blood lead levels higher than 70 [micro]g per dL, with or without encephalopathy, should be hospitalized and started on immediate chelation therapy. The CDC and the AAP recommend combined treatment with CaNa2EDTA and BAL.(16,26)
The recommended dosage of BAL is 25 mg per kg per day given in divided doses every four hours. The first dose of BAL should be administered at least four hours before the first dose of CaNa2EDTA because CaNa2EDTA alone may aggravate symptoms and BAL chelates the lead in brain tissue. The dosage of CaNa2EDTA is 50 mg per kg per day in a single dose given intravenously over several hours or by continuous infusion.(16,26)
The CDC recommends initial treatment with both BAL and CaNa2EDTA for five days. Blood lead levels should be monitored after this treatment. A second course of CaNa2EDTA alone is recommended if blood lead levels rebound to 45 to 69 [micro]g per dL; the use of CaNa2EDTA in combination with BAL is recommended for rebound blood lead levels higher than 70 [micro]g per dL. The CDC recommends waiting five to seven days between the end of the first course and the beginning of a second course. After another five-day waiting period, a third course of CaNa2EDTA alone may be needed if blood lead levels rebound to higher than 45 [micro]g per dL within 48 hours of the second course.(16)
The AAP recommendations differ slightly. According to the AAP, BAL can be stopped after a minimum of three days of initial treatment. CaNa2EDTA is then continued alone for the full five-day course.(26)
Challenges in Lead Poisoning Management
The management of lead poisoning remains challenging. Lead has a long half-life and is absorbed into bone and other tissues. Post-treatment rebound of blood lead levels is problematic, and questions remain about the duration of chelation therapy that is necessary to provide a clinically meaningful reduction in the overall body lead burden. Only one study,(27) using CaNa2EDTA, has shown a modest increase in IQ six months after treatment, and this outcome has not been demonstrated for all chelation agents. Other neurobehavioral outcomes of treatment also need to be studied. The CDC has emphasized the importance of addressing these challenges and has called for future research in the area of lead poisoning management.(5)
The authors thank Susan Kauffman, University of Missouri-Columbia, for assisting in the preparation of the manuscript.
REFERENCES
(1.) Rosen JF. Adverse health effects of lead at low exposure levels: trends in the management of childhood lead poisoning. Toxicology 1995;97:11-7.
(2.) Pirkle JL, Brody DJ, Gunter EW, Kramer RA, Paschal DC, Flegal KM, et al. The decline in blood lead levels in the United States. The National Health and Nutrition Examination Surveys (NHANES). JAMA 1994;272:284-91.
(3.) Trachtenbarg DE. Getting the lead out: when is treatment necessary? Postgrad Med 1996;99:201-2, 207-18.
(4.) Screening for elevated blood lead levels. American Academy of Pediatrics Committee on Environmental Health. Pediatrics 1998;101:1072-8.
(5.) Screening young children for lead poisoning: guidance for state and local public health officials. Atlanta: Centers for Disease Control and Prevention, National Center for Environmental Health, U.S. Dept. of Health and Human Services, Public Health Service, 1997.
(6.) Mendelsohn AL, Dreyer BP, Fierman AH, Rosen CM, Legano LA, Kruger HA, et al. Low-level lead exposure and behavior in early childhood. Pediatrics 1998;101:E10.
(7.) Dietrich KN, Berger OG, Succop PA. Lead exposure and the motor developmental status of urban six-year-old children in the Cincinnati Prospective Study. Pediatrics 1993;91:301-7.
(8.) Finkelstein Y, Markowitz ME, Rosen JF. Low-level lead-induced neurotoxicity in children: an update on central nervous system effects. Brain Res Brain Res Rev 1998;27:168-76.
(9.) Wright RO, Shannon MW, Wright RJ, Hu H. Association between iron deficiency and low-level lead poisoning in an urban primary care clinic. Am J Public Health 1999;89:1049-53.
(10.) Glotzer DE, Weitzman M, Aschengrau A, Freedberg KA. Economic evaluation of environmental interventions for low-level childhood lead poisoning. Ambul Child Health 1997;3:255-67.
(11.) Rooney BL, Hayes EB, Allen BK, Strutt PJ. Development of a screening tool for prediction of children at risk for lead exposure in a midwestern clinical setting. Pediatrics 1994;93:183-7.
(12.) Schaffer SJ, Szilagyi PG, Weitzman M. Lead poisoning risk determination in an urban population through the use of a standardized questionnaire. Pediatrics 1994;93:159-63.
(13.) American Academy of Family Physicians. Summary of recommendations for periodic health examination. Retrieved March 7, 2000, from the World Wide Web: http://www.aafp.org/exam/app-d5.html.
(14.) Tips NM, Falk H, Jackson RJ. CDC's lead screening guidance: a systematic approach to more effective screening. Public Health Rep 1998;113:47-51.
(15.) Berney B. Round and round it goes: the epidemiology of childhood lead poisoning, 1950-1990. Milbank Q 1993;71:3-39.
(16.) Centers for Disease Control. Preventing lead poisoning in young children: a statement by the Centers for Disease Control--October, 1991. 4th rev. Atlanta: U.S. Dept. of Health and Human Services, Public Health Service, Centers for Disease Control, 1991.
(17.) Kimbrough RD, LeVois M, Webb DR. Management of children with slightly elevated blood lead levels. Pediatrics 1994;93:188-91.
(18.) Hilts SR, Hertzman C, Marion SA. A controlled trial of the effect of HEPA vacuuming on childhood lead exposure. Can J Public Health 1995;86:345-50.
(19.) Lanphear BP, Winter NL, Apetz L, Eberly S, Weitzman M. A randomized trial of the effect of dust control on children's blood lead levels. Pediatrics 1996;98:35-40.
(20.) Rhoads GG, Ettinger AS, Weisel CP, Buckley TJ, Goldman KD, Adgate J, et al. The effect of dust lead control on blood lead in toddlers: a randomized trial. Pediatrics 1999;103:551-5.
(21.) Aschengrau A, Beiser A, Bellinger D, Copenhafer D, Weitzman M. The impact of soil lead abatement on urban children's blood lead levels: phase II results from the Boston Lead-In-Soil Demonstration Project. Environ Res 1994;67:125-48.
(22.) Weitzman M, Aschengrau A, Bellinger D, Jones R, Hamlin JS, Beiser A. Lead-contaminated soil abatement and urban children's blood lead levels. JAMA 1993;269:1647-54.
(23.) Swindell SL, Charney E, Brown MJ, Delaney J. Home abatement and blood lead changes in children with class III lead poisoning. Clin Pediatr [Phila] 1994;33:536-41.
(24.) Staes C, Matte T, Copley CG, Flanders D, Binder S. Retrospective study of the impact of lead-based paint hazard remediation on children's blood lead levels in St. Louis, Missouri. Am J Epidemiol 1994; 139:1016-26.
(25.) Amitai Y, Brown MJ, Graef JW, Cosgrove E. Residential deleading: effects on the blood lead levels of lead-poisoned children. Pediatrics 1991;88:893-7.
(26.) Treatment guidelines for lead exposure in children. American Academy of Pediatrics Committee on Drugs. Pediatrics 1995;96:155-60.
(27.) Ruff HA, Bijur PE, Markowitz M, Ma YC, Rosen JF. Declining blood lead levels and cognitive changes in moderately lead-poisoned children. JAMA 1993;269:1641-6.
(28.) Sachs HK, Blanksma LA, Murray EF, O'Connell MJ. Ambulatory treatment of lead poisoning: report of 1,155 cases. Pediatrics 1970;46:389-96.
(29.) Besunder JB, Anderson RL, Super DM. Short-term efficacy of oral dimercaptosuccinic acid in children with low to moderate lead intoxication. Pediatrics 1995;96:683-7.
(30.) Liebelt EL, Shannon M, Graef JW. Efficacy of oral meso-2,3-dimercaptosuccinic acid therapy for low-level childhood plumbism. J Pediatr 1994;124:313-7.
(31.) Besunder JB, Super DM, Anderson RL. Comparison of dimercaptosuccinic acid and calcium disodium ethylenediaminetetraacetic acid versus dimercaptopropanol and ethylenediaminetetraacetic acid in children with lead poisoning. J Pediatr 1997;130: 966-71.
(32.) Graziano JH, Lolacono NJ, Moulton T, Mitchell ME, Slavkovich V, Zarate C. Controlled study of meso-2,3-dimercaptosuccinic acid for the management of childhood lead intoxication. J Pediatr 1992; 120:133-9.
(33.) Graziano JH, Lolacono NJ, Meyer P. Dose-response study of oral 2,3-dimercaptosuccinic acid in children with elevated blood lead concentrations. J Pediatr 1988;113:751-7.
(34.) O'Connor ME, Rich D. Children with moderately elevated lead levels: is chelation with DMSA helpful? Clin Pediatr [Phila] 1999;38:325-31.
(35.) The Treatment of Lead-exposed Children (TLC) trial: design and recruitment for a study of the effect of oral chelation on growth and development in toddlers. Paediatr Perinat Epidemiol 1998; 12:313-33.
(36.) Shannon M, Graef J, Lovejoy FH. Efficacy and toxicity of D-penicillamine in low-level lead poisoning. J Pediatr 1988;112:799-804.
Members of various medical faculties develop articles for "Practical Therapeutics." This article is one in a series coordinated by the Department of Family and Community Medicine at the University of Missouri-Columbia School of Medicine, Columbia, Mo. Guest editor of the series is Robert L. Blake, Jr., M.D.
MARK R. ELLIS, M.D., M.S.P.H., is faculty physician in the family practice residency program of Cox Health Systems, Springfield, Mo. Previously, he was clinical instructor and academic fellow in the Department of Family and Community Medicine at the University of Missouri-Columbia School of Medicine, where he also completed a family practice residency. Dr. Ellis received his medical degree from the University of Arkansas College of Medicine and completed a pediatric internship at Arkansas Children's Hospital, both in Little Rock.
KEVIN Y. KANE, M.D., M.S.P.H., is assistant professor in the Department of Family and Community Medicine at the University of Missouri-Columbia School of Medicine. He received his medical degree from Creighton University School of Medicine, Omaha, and completed a family medicine residency and an academic fellowship at the University of Missouri-Columbia.
Address correspondence to Mark R. Ellis, M.D., M.S.P.H., Family Practice Residency, Cox Health Systems, 1423 N. Jefferson, A100, Springfield, MO 65802. Reprints are not available from the authors.
COPYRIGHT 2000 American Academy of Family Physicians
COPYRIGHT 2000 Gale Group