Drug eruptions are common iatrogenic diseases. Although most of these conditions are benign and self-limited when use of the responsible drug is discontinued, several subtypes of drug eruptions are characterized by significant morbidity and mortality. The more dangerous types include erythroderma, leukocytoclastic vasculitis, anticonvulsant hypersensitivity syndrome, Stevens-Johnson syndrome and toxic epidermal necrolysis. Relatively few medications are repeatedly implicated in the pathogenesis of severe reactions. Prompt recognition of drug eruptions and early intervention are necessary to prevent the serious consequences of this group of iatrogenic diseases.
As options for pharmacotherapy continue to expand, so does the frequency of iatrogenic disease. Adverse reactions to medications are common, with a reported incidence of 10 to 20 percent in hospitalized patients.(1)(2)(3)(4)(5) Although less well documented, the rate of outpatient visits related to adverse drug reactions has been estimated to be as high as one per 40 visits to general practitioners.(1)
Cutaneous eruptions are one of the most frequent presentations of adverse drug reactions. Maculopapular, morbilliform and urticarial eruptions are the most common types and usually resolve without incident when the responsible medication is discontinued.(6)(7)(8) However, several less common cutaneous reactions are associated with significant morbidity and mortality.(1)(6)(9) Medications that are most frequently implicated in serious drug eruptions are listed in Table 1.
TABLE 1 Medications That Can Cause Serious Cutaneous Drug Eruptions
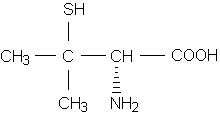
An awareness of cross-reactivity between medications is important, since patients often take multiple drugs concurrently and more than one medication may be responsible for the reaction. An example of this situation involves the diverse thiol (sulfhydryl-containing) medications (Table 1), which are often used simultaneously for unrelated indications.
Prompt recognition and early intervention are critical in minimizing the morbidity and mortality associated with severe cutaneous drug reactions.
Erythroderma
Erythroderma, or exfoliative dermatitis, is defined as diffuse inflammation of all or most of the cutaneous surface. Erythroderma has a male predominance and an average age of onset in the sixth decade.(10)(11) The etiology of erythroderma generally falls into one of five categories: (1) preexisting dermatoses (e.g., psoriasis), (2) drug eruptions, (3) underlying malignancy, (4) miscellaneous factors (e.g., acquired immunodeficiency syndrome) and (5) idiopathic causes.(10)(11)(12) Drug-induced erythroderma accounts for 10 to 20 percent of cases in most series.(11)(12) Antiepileptic, antihypertensive and antibiotic medications are frequently implicated, but many drugs, including topical preparations, may be a possible cause.(10)(11)
Exfoliative dermatitis presents as generalized erythema and desquamation (Figure 1), often associated with pruritus, fever and chills.(10)(11) Lymphadenopathy is frequently noted, and hepatosplenomegaly is present in a minority of patients.(10)(11) Complications of exfoliative dermatitis may include high-output cardiac failure due to expanded circulation, hypothermia and hyperpyrexia, and a negative nitrogen balance.(13)
[ILLUSTRATION OMITTED]
Leukocytosis, eosinophilia, mild anemia and abnormal serum protein electrophoresis may be present.(10)(11)(13) Skin biopsy often has nonspecific findings but may reveal a preexisting dermatosis or show changes consistent with mycosis fungoides.(10)
The mechanism by which erythroderma develops in patients using topical medications is an irritant or allergic contact dermatitis. The pathogenesis of exfoliative dermatitis secondary to systemic medications is less clear.(10)
Treatment of drug-induced erythroderma includes discontinuation of the medication, daily baths, emollients, antihistamines and topical or systemic corticosteroids.(10)(13) Although the reported mortality rates for erythroderma have ranged from 8 percent to 64 percent, patients with drug-induced erythroderma have a much more favorable prognosis, with full recovery in the majority of cases.(10)(13)
Leukocytoclastic Vasculitis
Leukocytoclastic vasculitis, or allergic vasculitis, is the most common of the serious drug eruptions. Although leukocytoclastic vasculitis may have several etiologies, the factors most frequently implicated are medications, infections and immune-complex diseases.(14)(15)
The eruption of vasculitis usually begins in dependent areas, such as the lower extremities in ambulatory patients or the posterior thorax and sacrum in nonambulatory patients. The first lesions to appear are blanching erythematous macules, which may rapidly become elevated and purpuric (Figure 2), and occasionally bullous. Pruritus, pain and dependent edema are variably present. Systemic signs such as fever, arthralgias, myalgias, arthritis and abdominal pain may occur.(16) In severe cases, vasculitis may affect renal, gastrointestinal, pulmonary, cardiac and central nervous systems.(16)
[ILLUSTRATION OMITTED]
Biopsy of an early skin lesion shows neutrophilic infiltration and fibrinoid necrosis of the walls of blood vessels, as well as leukocytoclasia.(14)(16) Direct immunofluorescence most commonly reveals deposition of IgM, complement or fibrin around dermal blood vessels.(14) Laboratory abnormalities may include hematuria, proteinuria and uremia, as well as elevated serum creatinine levels and erythrocyte sedimentation rates.
Leukocytoclastic vasculitis is a classic type III hypersensitivity reaction, in which immune complexes are deposited in postcapillary venules, with resultant complement activation and neutrophil recruitment.(14)(16) Release of lysosomal enzymes from neutrophils leads to vessel damage and the resultant clinicopathologic findings.
Many medications have been implicated in the etiology of leukocytoclastic vasculitis, but some drugs cause this reaction more frequently.(1)(16) These drugs include penicillins, thiazide diuretics, sulfonamides, nonsteroidal anti-inflammatory drugs (NSAIDs) and quinidine.(1)(16)
The evaluation of a patient with leukocytoclastic vasculitis should include a complete history, physical examination and laboratory tests, including a complete blood count, assessment of renal function, urinalysis and stool testing for occult blood. Further testing, as dictated by the preliminary clinical or laboratory findings, may be appropriate if no drug is implicated.
Potentially responsible medications must be promptly discontinued. For patients with extracutaneous involvement, treatment with systemic corticosteroids, azathioprine (Imuran), methotrexate or cyclophosphamide (Cytoxan) may be necessary.(15)(16) Patients whose vasculitis is limited to the skin may need only topical care or may require treatment with systemic corticosteroids, sulfones, colchicine or, rarely, azathioprine.(15)(16) Prognosis is related to the presence or absence of systemic involvement.
Anticonvulsant Hypersensitivity Syndrome
The anticonvulsant hypersensitivity syndrome, also known as the phenytoin (Dilantin) pseudolymphoma syndrome, is a rare complication that occurs with the use of antiepileptic medications, most commonly phenytoin. Carbamazepine (Tegretol) and phenobarbital have also been reported to cause a similar reaction.(17)(18)
In most cases, hypersensitivity develops three weeks to three months after initiation of therapy, which is much later than the time at which is much later than the time at which most drug reactions occur.(19)(20) The cardinal features of anticonvulsant hypersensitivity syndrome are high spiking fever, rash, lymphadenopathy and hepatitis.(17)(19)(20) Although a maculopapular erythematous eruption (Figure 3) is the most common cutaneous presentation, diffuse pustulation or erythroderma is not uncommon.(17) Edema, especially facial or acral, is a frequent finding (Figure 4).(17)(18) Lymphadenopathy may be localized or diffuse.(20)
[ILLUSTRATION OMITTED]
Laboratory investigations commonly reveal leukocytosis with frequent eosinophilia, as well as elevated serum transaminase values. Histopathology of skin and lymph nodes may vary from a reactive pattern to frank lymphomatous changes.(19)(20)(21)
It has been postulated that this syndrome occurs as a result of a delayed hypersensitivity reaction.(17)(22) Treatment includes discontinuation of the medication, with careful substitution necessary because of the high rate of cross-reactivity between anticonvulsant medications.(17)(18)(20) Systemic corticosteroids have been anecdotally reported to be effective in more extensive cases of anticonvulsant hypersensitivity syndrome.(17)
Prognosis is variable. The two most important factors affecting prognosis are hepatic involvement and the relationship of the syndrome with lymphoma.(17)(19)(21) In the presence of a toxic hepatitis, the mortality rate has been reported to range from 10 to 50 percent.(17) Most patients with nodal histologic features of lymphoma have regression of malignant features when the drug is discontinued(19)(21); these patients have "pseudolymphoma." In a subset of patients with "pseudopseudolymphoma," however, malignant lymphoma develops within months to years despite the occurrence of short-term clinical and histologic regression of the disease after cessation of the medication.(19)(21) A small subgroup of patients have malignant lymphoma that is present at the time of the onset of the anticonvulsant hypersensitivity syndrome and persists.(19)
Stevens-Johnson Syndrome
Stevens-Johnson syndrome, or erythema multiforme major, represents the more severe expression of erythema multiforme, as originally described by Hebra.(23) Erythema multiforme minor, characterized by cutaneous "target" lesions and limited mucosal involvement, is usually managed on an outpatient basis. Stevens-Johnson syndrome, however, is associated with significant morbidity and mortality, as well as a drug-induced etiology in the majority of cases.(23)
Although Stevens-Johnson syndrome is most common in the first three decades of life, it may present at any age.(23)(24)(25) A male predominance has been noted.(23)(24) Most cases of the syndrome are related to use of medications, particularly sulfonamides, penicillins, barbiturates and anticonvulsant medications.(23)(26) Mycoplasma pneumoniae, herpes simplex and Streptococcus species are among the more well-established infectious causes of Stevens-Johnson syndrome.(23)(24)(27)
Stevens-Johnson syndrome usually begins with a prodrome suggestive of an upper respiratory tract infection, with fever, cough and malaise.(23)(24) One to 14 days later, the characteristic features develop.(23) Cutaneous manifestations include erythematous papules and plaques, which rapidly develop a dusky center--"target" lesion (Figure 5)--or a bullous center--"iris" lesion.(23)(24)(27) The trunk, face and distal extremities are most commonly involved.(23)
Involvement of at least two mucous membranes is necessary for diagnosis of Stevens-Johnson syndrome, since more mild involvement of a single mucosal surface may occur with erythema multiforme minor.(25)(26) Most frequently affected are the oropharyngeal mucosa (bullae, ulcerative stomatitis, hemorrhagic cheilitis [Figure 6], gingivitis) and ocular mucosa (conjunctivitis, blepharitis, ulceration), although genitourinary, gastrointestinal and tracheobronchial mucous membranes may also be involved.(23)(24)(28) In patients with severe oral involvement inadequate fluid intake may result, leading to dehydration and electrolyte imbalance.(27)
[ILLUSTRATION OMITTED]
The clinical course of Stevens-Johnson syndrome may be complicated by pneumonia, esophageal strictures, hepatitis, nephritis and septicemia.(23)(25) Potential ocular sequelae include trichiasis, corneal ulceration and blindness.(23)(27)
Leukocytosis, uremia, electrolyte imbalances and an elevated erythrocyte sedimentation rate may be present, but all of these findings are nonspecific.(24) Skin biopsy reveals perivascular mononuclear cells, dermal edema and necrotic keratinocytes; formation of subepidermal bullae may be seen in more severely affected areas.(29)
The precise pathophysiology of Stevens-Johnson syndrome is unknown. However, a hypersensitivity reaction is widely believed to be the common link between the various etiologies of Stevens-Johnson syndrome.(23)(25)
The principal differential diagnosis for Stevens-Johnson syndrome is toxic epidermal necrolysis. Although sometimes considered to be a more extreme expression of Stevens-Johnson syndrome, toxic epidermal necrolysis is distinguished from Stevens-Johnson syndrome by its rapid evolution (24 to 48 hours), more extensive bullae and erosions (affecting more than 20 percent of the body surface), usual absence of target lesions and histology characterized by full-thickness epidermal necrosis with a sparse dermal inflammatory infiltrate.(25)(30)
The treatment of Stevens-Johnson syndrome includes hospitalization in most cases, with attention to fluid status, electrolytes and pain control. Local skin care is best managed by use of whirlpool, application of saline dressings for weeping or bullous areas and use of topical antibiotics (although silver sulfadiazine [Silvadene] should be avoided because of its sulfur moiety).(23) Viscous lidocaine or diphenhy-dramine (Benadryl) elixir may be helpful for the pain associated with oral ulceration.(23) Consulatation with an ophthalmologist is important to prevent ocular sequelae.
Systemic corticosteroids are sometimes used for the treatment of medication-related Stevens-Johnson syndrome,(23) but use of these drugs has been associated with an increased mortality rate due to more frequent infectious complications, gastrointestinal hemorrhage and delayed healing.(24)(27)(28)(31) The overall rate of mortality from Stevens-Johnson syndrome ranges from 5 to 25 percent, with death usually related to infectious complications.(23)(25)
Toxic Epidermal Necrolysis
Toxic epidermal necrolysis is the most serious of all drug eruptions. The reported mortality rate for patients with this syndrome ranges from 11 to 70 percent.(32)(33) Its precise nosology remains unclear, since some authors have suggested a relationship of this syndrome with Stevens-Johnson syndrome, while others have argued for its classification as a distinct entity.(30) For the sake of clarity, toxic epidermal necrolysis is discussed separately in this article because of its dramatic clinical course and grave prognosis.
Toxic epidermal necrolysis most commonly occurs in adults, but it has been reported in neonates and children.(32) It is slightly more common in females than in males, and the annual incidence has been estimated as approximately one case per 1 million persons.(34)
Although toxic epidermal necrolysis occasionally has been linked with immunizations, malignancies and infections, the overwhelming majority of cases are drug-induced.(32)(33)(34) Among the most commonly implicated medications are NSAIDs--especially piroxicam (Feldene) and phenylbutazone--and sulfonamides, penicillins, anticonvulsants (phenytoin, phenobarbital and carbamazepine), allopurinol (Zyloprim) and salicylates.(25)(32)(33)(34) In India, a large proportion of cases of toxic epidermal necrolysis have occurred in association with the use of isoniazid (Laniazid, Nydrazid), which suggests that vigilance for isoniazid-related toxic epidermal necrolysis would be appropriate given the worldwide resurgence of tuberculosis.(33)
Clinically, toxic epidermal necrolysis is characterized by a febrile prodrome that resembles an upper respiratory tract infection.(33) A diffuse morbilliform eruption or confluent erythema usually follows the prodrome.(30) This in turn leads into the acute phase of toxic epidermal necrolysis, characterized by persistent fever, prominent mucous membrane involvement and generalized epidermal sloughing (Figure 7).(32)(33)(34) Not infrequently, patients may lose all of thier epidermis within 24 hours.(33) Although ocular and oral involvement is often the most prominent, any mucous membrane, including the tracheobronchial, gastrointestinal and genitourinary mucous membranes, may be involved.(32)(33)
[ILLUSTRATION OMITTED]
Systemic involvement frequently occurs in patients with toxic epidermal necrolysis. Pneumonia commonly occurs as a result of aspiration of sloughed tracheobronchial mucosa.(25)(33) Hypovolemia and electrolyte imbalances often occur in the absence of a cutaneous barrier to water loss and coupled with poor oral intake. Massive gastrointestinal hemorrhage may occur.(33) Septicemia is the most frequent cause of death in patients with toxic epidermal necrolysis, and it is usually due to Staphylococcus aureus or Pseudomonas from skin, lungs or indwelling catheters.(25)(33) Long-term sequelae in survivors of toxic epidermal necrolysis may involve cutaneous problems (hypohidrosis, contractures, scarring), mucosal damage (xerostomia, phimosis, esophageal strictures) or ocular complications (corneal ulceration, symblepharon, blindness).(25)(32)(33)
Laboratory evaluation may reveal leukopenia, thrombocytopenia, anemia, hypoalbuminemia, hypocalcemia and severe hypophosphatemia.(32) Skin biopsy shows a subepidermal blister with overlying full-thickness epidermal necrosis and an underlying dermis that is usually remarkable for the paucity of inflammation.(32)(33)
Although several hypotheses have been advanced, the pathophysiology of toxic epidermal necrolysis remains unknown. The belief that this syndrome is an immunologic disease is the most popular concept, but it is not universally accepted.(35) Nonimmunologic mechanisms, such as direct necrosis of keratinocytes by toxic drug metabolites in genetically susceptible persons, have also been proposed.(32)
Treatment of toxic epidermal necrolysis includes hospitalization in a burn unit, debridement of necrotic epidermis, application of synthetic dressings, skin grafting of denuded areas and careful monitoring of fluid and electrolytes. Frequent skin and blood cultures, frequent changing of indwelling catheters and prompt institution of antibiotics if signs of infection appear are necessary to avoid the high mortality rate associated with sepsis. Topical antibiotics are universally used. However, as in patients with Stevens-Johnson syndrome, use of silver sulfadiazine should be avoided because of the frequent association of sulfonamides with the induction of toxic epidermal necrolysis. Ophthalmologic consultation is important to lyse adhesions and help prevent ocular complications. Systemic corticosteroids are generally believed to be contraindicated in the treatment of toxic epidermal necrolysis, because of potential infectious complications,(31)(33) although this contraindication is not uniformly accepted.(35)
Adverse prognostic factors that have been proposed include advanced age, elevated blood urea nitrogen and extensive necrolysis.(32)(33) Multiple drug ingestion, sepsis, leukopenia and thrombocytopenia are also factors that have been suggested to portend a poorer outcome in patients with toxic epidermal necrolysis.(32)(33)
REFERENCES
(1.)Breathnach SM, Hintner H, eds. Adverse drug reactions and the skin. Boston: Blackwell Scientific Publications, 1992.
(2.)Simmons M, Parker JM, Gowdy CW, Coulter WK. Adverse drug reactions during hospitalization [Letter]. Can Med Assoc J 1968; 98:175.
(3.)Smidt NA, McQueen EG. Adverse reactions to drugs: a comprehensive hospital inpatient survey. N Z Med J 1972; 76:397-401.
(4.)Kellaway GS, McCrae E. Intensive monitoring for adverse drug effects in patients discharged from acute medical wards. N Z Med J 1973; 78:525-8.
(5.)Gardner P, Watson LJ. Adverse drug reactions: a pharmacist-based monitoring system. Clin Pharmacol Ther 1970; 11:802-7.
(6.)Wintroub BU, Stern R. Cutaneous drug reactions: pathogenesis and clinical classification. J Am Acad Dermatol 1985; 13(2 Pt 1):167-79.
(7.)O'Brien TJ. Cutaneous reactions to drugs. Aust Fam Physician 1986; 15:861-5.
(8.)Goolamali SK. Drug eruptions. Postgrad Med J 1985; 61:925-33.
(9.)Millikan LE. Recognizing drug-related skin eruptions. Drug Ther 1993; 23:27-39.
(10.)Wilson DC, Jester JD, King LE Jr. Erythroderma and exfoliative dermatitis. Clin Dermatol 1993; 11:67-72.
(11.)Hasan T, Jansen CT. Erythroderma: a follow-up of fifty cases. J Am Acad Dermatol 1983; 8:836-40.
(12.)Thestrup-Pedersen K, Halkier-Sorensen L, Sogaard H, Zachariae H. The red man syndrome. Exfoliative dermatitis of unknown etiology: a description and follow-up of 38 patients. J Am Acad Dermatol 1988; 18:1307-12.
(13.)Marks J. Erythroderma and its management. Clin Exp Dermatol 1982; 7:415-22.
(14.)Gibson LE. Cutaneous vasculitis: approach to diagnosis and systemic associations. Mayo Clin Proc 1990; 65:221-9.
(15.)Callen JP. Cutaneous vasculitis and other neutrophilic dermatoses. Curr Opin Rheumatol 1993; 5:33-40.
(16.)Jorizzo JL. Classification of vasculitis. J Invest Dermatol 1993; 100:106S-10S.
(17.)Kleier RS, Breneman DL, Boiko S. Generalized pustulation as a manifestation of the anticonvulsant hypersensitivity syndrome. Arch Dermatol 1991; 127:1361-4.
(18.)Rivey MP, Stone JD. Carbamazepine hypersensitivity reaction. Brain Inj 1991; 5:57-62.
(19.)Harris DW, Ostlere L, Buckley C, Whittaker S, Sweny P, Rustin MH. Phenytoin-induced pseudolymphoma. A report of a case and review of the literature. Br J Dermatol 1992; 127:403-6.
(20.)Silverman AK, Fairley J, Wong RC. Cutaneous and immunologic reactions to phenytoin. J Am Acad Dermatol 1988; 18(4 Pt 1):721-41.
(21.)D'Incan M, Souteyrand P, Bignon YJ, Fonck Y, Roger H. Hydantoin-induced cutaneous pseudolymphoma with clinical, pathologic, and immunologic aspects of Sezary syndrome. Arch Dermatol 1992; 128:1371-4.
(22.)Leeder JS, Riley RJ, Cook VA, Spielberg SP. Human anti-cytochrome P450 antibodies in aromatic anticonvulsant-induced hypersensitivity reactions. J Pharmacol Exp Ther 1992; 263:360-7.
(23.)Araujo OE, Flowers FP. Stevens-Johnson syndrome. J Emerg Med 1984; 2:129-35.
(24.)Levy M, Shear NH. Mycoplasma pneumoniae infections and Stevens-Johnson syndrome. Report of eight cases and review of the literature. Clin Pediatr 1991; 30:42-9.
(25.)Leenutaphong V, Sivayathorn A, Suthipinittharm P, Sunthonpalin P. Stevens-Johnson syndrome and toxic epidermal necrolysis in Thailand. Int J Dermatol 1993; 32:428-31.
(26.)Chan HL, Stern RS, Arndt KA, Langlois J, Jick SS, Jick H, et al. The incidence of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. A population-based study with particular reference to reactions caused by drugs among outpatients. Arch Dermatol 1990; 126:43-7.
(27.)Stitt VJ Jr. Stevens-Johnson syndrome: a review of the literature. J Natl Med Assoc 1988; 80:104,106-8.
(28.)Edell DS, Davidson JJ, Muelenaer AA, Majure M. Unusual manifestation of Stevens-Johnson syndrome involving the respiratory and gastrointestinal tract. Pediatrics 1992; 89:429-32.
(29.)Lever WF, Schaumburg-Lever G. Noninfectious vesicular and bullous diseases. In: Lever WF, Schaumburg-Lever G. Histopathology of the skin. 7th ed. Philadelphia: Lippincott, 1990:135-8.
(30.)Goldstein SM, Wintroub BW, Elias PM, Wuepper KD. Toxic epidermal necrolysis. Unmuddying the waters. Arch Dermatol 1987; 123:1153-6.
(31.)Halebian PH, Shires GT. Burn unit treatment of acute, severe exfoliating disorders. Annu Rev Med 1989; 40:137-47.
(32.)Parsons JM. Toxic epidermal necrolysis. Int J Dermatol 1992; 31:749-68.
(33.)Avakian R, Flowers FP, Araujo OE, Ramos-Caro FA. Toxic epidermal necrolysis: a review. J Am Acad Dermatol 1991; 25(1 Pt 1):69-79.
(34.)Roujeau JC, Guillaume JC, Fabre JP, Penso D, Flechet ML, Girre JP. Toxic epidermal necrolysis (Lyell syndrome). Incidence and drug etiology in France, 1981-1985. Arch Dermatol 1990; 126:37-42.
(35.)Revuz J, Roujeau JC, Guillaume JC, Penso D, Touraine R. Treatment of toxic epidermal necrolysis. Creteil's experience. Arch Dermatol 1987; 123:1156-8.
COPYRIGHT 1995 American Academy of Family Physicians
COPYRIGHT 2004 Gale Group