Study objectives: Treatment of patients with idiopathic pulmonary fibrosis (IPF) conventionally includes corticosteroids and cytotoxic agents. No study to date has adequately evaluated the benefits of this approach. This study retrospectively compared combination corticosteroid and cyclophosphamide therapy in a large population of patients who meet the current consensus definition of IPF.
Design: Patients were identified retrospectively and treatment addressed on an intention-to-treat basis. Treated and untreated patients were matched by age and percentage of predicted FVC (FVC%) at the time of the initial visit.
Setting: Two academic tertiary referral centers.
Patients or participants: The diagnosis of IPF was based on current consensus criteria. A total of 164 patients (82 treated and 82 untreated) were included.
Interventions: Treatment consisted of combined corticosteroid and cyclophosphamide therapy using a standardized protocol.
Measurements and results: There was no difference in age, FVC%, gender, or smoking status between groups. No survival difference was found between patients who were treated (median survival, 1,431 days) or untreated (median survival, 1,665 days) [p = 0.58]. The lack of treatment effect persisted when only those patients with a diagnosis by surgical biopsy (n = 24) or FVC% [greater than or equal to] 60 (n = 107) were analyzed.
Conclusions: Our data suggest that combined corticosteroid and cyclophosphamide therapy has no impact on survival in patients with IPF. This finding supports the evolving concept that chronic inflammation plays a minimal role in the progression of IPF and reinforces the importance of careful consideration of the risks and benefits of such therapies prior to their institution.
Key words: corticosteroid; cyclophoshamide; cytotoxic: idiopathic pulmonary fibrosis; prognosis; pulmonary fibrosis; survival; therapeutics; treatment
Abbreviations: ATS/ERS = American Thoracic Society and European Respiratory Society; FVC% = percentage of predicted FVC; IPF = idiopathic pulmonary fibrosis
**********
Idiopathic pulmonary fibrosis (IPF), the most common of the idiopathic interstitial pneumonias, affects up to a million people worldwide. The diagnosis of IPF has been recently redefined by the American Thoracic Society and European Thoracic Society (ATS/ERS), and requires specific clinical and radiologic features in conjunction with the histopathologic pattern of usual interstitial pneumonia. (1) Historically, published studies (2-5) of IPF have included patients with other histopathologic patterns (eg, nonspecific interstitial pneumonia, desquamative interstitial pneumonia, respiratory bronchiolitis-associated interstitial lung disease, cryptogenic organizing pneumonia, lymphocytic interstitial pneumonia) now believed to have distinct clinical behaviors, as noted in the ATS/ERS consensus statement on the classification of the idiopathic interstitial pneumonias. (6)
IPF has a uniquely poor prognosis among the nonacute idiopathic interstitial pneumonias, and is believed to be poorly responsive to currently available therapy. (7) The recommended treatment for patients in whom therapy is believed to be warranted is a combination of corticosteroids and cytotoxic therapy (either azathioprine or cyclophosphamide). (1) However, no randomized, placebo-controlled trial has been performed that addresses whether immunosuppressive therapy provides clinically significant benefit when compared with no therapy in patients who meet the current definition of IPF. To date, published studies have been largely confounded by changing case definitions of IPF, poorly matched treated and untreated populations, and small numbers of patients. With these limitations, several prospective studies have compared combination therapy with corticosteroids and cytotoxic agents to corticosteroids alone and have failed to show a statistically significant difference in morbidity or survival time. (8-17) In the present study, we compared survival in patients treated with the combination of corticosteroid and cyclophosphamide therapy to untreated patients matched for age at presentation and baseline percentage of predicted FVC (FVC%) in a large, well-defined population of patients with IPF.
MATERIALS AND METHODS
Study Population
Patients seen at two institutions (National Jewish Medical and Research Center, Denver, CO and the Mayo Clinic, Rochester, MN) between 1984 and 2002 who received a diagnosis of IPF were retrospectively reviewed. Treated patients (n = 543) and untreated patients (n = 155) were matched by age at diagnosis (within 5 years) and baseline FVC% (within 5%). This identified 164 patients (82 treated/untreated matched patient pairs) who comprised the study group. The diagnosis of IPF was made by surgical lung biopsy in 57% of these patients (n = 93). An expert pulmonary pathologist had reviewed all surgical lung biopsies and confirmed the presence of a usual interstitial pneumonia pattern using current histopathologic criteria. All patients who did not undergo surgical lung biopsy (n = 71) met the current clinical and radiologic criteria for the presumptive diagnosis of IPF as detailed in the recent ATS/ERS consensus statement on IPF, (1) or had a clinicoradiographic presentation believed to be highly specific for IPF. (18) Baseline measurements of FVC% were defined as those measured at the time of initial visit to our respective institutions. Subjects were designated current smokers (having smoked within the previous year), former smokers (not having smoked within the previous year but have a smoking history), or never-smokers. Informed consent was obtained from each patient, and both institutional human subject review committees approved the protocol.
Treatment Protocol
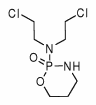
All treated patients were from National Jewish Medical and Research Center. Treatment was generally determined by standard treatment outline. Recommended treatment consisted of corticosteroids (initial starting dose, 0.5 to 1.0 mg/kg of prednisone or its equivalent) with the addition of cyclophosphamide (2 mg/kg/d po with a total dose < 200 mg/d). Corticosteroids were tapered to lower doses after several weeks and continued for at least 6 months and commonly 12 months, unless significant adverse drug effects occurred. All untreated patients were from the Mayo Clinic, Rochester, MN.
Survival Analysis
All analyses were performed on an intention-to-treat basis. Survival time was calculated from the initial visit until death or time of censoring. Patients were censored if they were still alive at last contact. Vital status was ascertained by follow-up with the patient, the patient's family or personal physician, or by searching the national death registry. To compare survival between treated and untreated groups, survival curves were estimated using the Kaplan-Meier approach; survival curves were compared using the log-rank test. This approach was used to compare survival in matched treated and untreated patients with a diagnosis by surgical lung biopsy (n = 24) and in treated and untreated patients with a baseline FVC% [greater than or equal to] 60% (n = 107). Cox proportional hazards regression was used to estimate the impact of treatment, baseline FVC%, smoking status, and surgical lung biopsy on mortality; each of these hazard ratios was adjusted for age. All data analyses were performed using SAS Version 8 (SAS Institute; Cary, NC).
RESULTS
Patient Characteristics
Treated and untreated patients were well matched for age at diagnosis, gender, baseline FVC%, and smoking history (Table 1). The overall mean age at diagnosis was 67.6 years. There were 127 men and 37 women, equally distributed between the two groups. Overall, baseline FVC% was 67.2. A history of current or former smoking was present in approximately 70% of patients. Surgical lung biopsy was obtained in all treated patients and in 12 of the untreated patients (15%).
Survival: Treated vs Untreated
There was no difference in survival from the time of initial visit between treated and untreated patients (Fig 1). Eighty-nine of time 164 patients died during the study period. Median follow-up was 679 days for treated patients and 1,079 days for untreated patients. Median survival was 1,431 days (95% confidence interval, 1,110 to 2,002 days) in treated patients and 1665 days (95% confidence interval, 1,126 to not calculable) in untreated patients (p = 0.58). The lack of a survival benefit with treatment persisted when survival analysis was limited to only those matched patient pairs who had undergone surgical lung biopsy (Fig 2), or patients who had a FVC% [greater than or equal to] 60 (Fig 3).
[FIGURES 1-3 OMITTED]
Cox Regression Analysis
Lack of survival benefit with combined corticosteroid add cyclophosphamide therapy persisted when the analysis was adjusted for the known or potential risk factors of age, baseline FVC%, diagnosis by surgical lung biopsy, and smoking status (Table 2). Of the additional variables analyzed, younger age and higher baseline FVC% were associated with improved survival time, and current smoking was associated with worse survival. Neither prior smoking nor surgical lung biopsy was associated with survival.
DISCUSSION
Combined treatment with corticosteroid and cyclophosphamide therapy does not appear to alter survival in patients with IPF. The lack of survival benefit persists when only surgical biopsy-proven cases or cases with a FVC% [greater than or equal to] 60 are analyzed. These findings support the concept that chronic persistent inflammation plays little role in the progression of IPF. (19-21)
Interpretation of previous studies addressing the impact of corticosteroid and cytotoxic therapy on survival is difficult and unreliable given varying case definitions of IPF, poorly matched treatment groups, and small numbers of patients. In 1980, Turner-Warwick and colleagues (11) retrospectively reviewed > 200 patients with IPF (termed cryptogenic fibrosing alveolitis) and found no statistical difference in survival between those patients treated with corticosteroid and those untreated, a finding that has been confirmed by several subsequent studies. (12-16) These studies all included unmatched patient populations and patients who would now be considered to have alternative forms of idiopathic interstitial pneumonia. Riha and colleagues (17) reviewed a population of largely surgical biopsy-proven IPF in which 27 patients received treatment with corticosteroid and cytotoxic therapy and 8 patients received no treatment. No difference in survival was seen (p = 0.14) between the two groups. Although this is a small and unmatched study, the findings support our results.
Several prospective studies (8,9,22) have combined corticosteroid and cytotoxic therapy in patients with IPF. Johnson and colleagues (8) published a randomized trial comparing prednisolone and cyclophosphamide to prednisolone alone in 43 patients with IPF. While the survival curves appeared to diverge, there was no statistical difference in measures of clinical condition or survival time with the addition of cyclophosphamide. Raghu and colleagues (9) subsequently published a randomized, placebo controlled trial comparing prednisone and azathioprine to prednisone alone in 27 patients with IPF. No survival advantage was found with the addition of azathioprine (hazard ratio, 0.48; 95% confidence interval, 0.17 to 1.38). After adjusting for age, a survival advantage became statistically significant, suggesting a possible benefit to combination therapy with azathioprine. Both of these studies likely included patients with alternative forms of idiopathic interstitial pneumonia that may be more responsive to anti-inflammatory therapy, and neither included untreated patients.
There are limitations to our current study. First, retrospective studies are always vulnerable to clinical variables not controlled or adjusted for in the analysis. We have matched patients for two of the most clearly predictive clinical variables, age and FVC%, in an attempt to control for potential confounding. Cox regression analysis was performed to adjust for additional variables. Second, not all untreated patients underwent surgical lung biopsy, raising the possibility of including alternative histopathologic patterns. To address this concern, we required that all patients who did not undergo biopsy meet strict clinical and radiologic criteria known to have an extremely high specificity for the diagnosis of IPF. (18,23) Analysis of only those untreated patients who underwent surgical biopsy showed no difference in survival compared to the matched treated cohort. Third, many patients with IPF have advanced disease on presentation, manifest by widespread fibrosis and honeycombing on surgical biopsy or high-resolution CT. These patients experience higher short-term mortality, (24) and their inclusion may mask any potential treatment effect in patients with less advanced disease. Using FVC% as a surrogate for disease severity, we analyzed only those patients with a FVC% [greater than or equal to] 60 and still found no difference in survival between the treated and untreated groups. Fourth, we did not evaluate changes in clinical symptoms, quality of life, or physiology with and without therapy. These important outcome measures may be affected by treatment. Finally, treated and untreated patients came from separate tertiary care institutions, raising the possibility that differences in local referral and management practices may have influenced survival.
A small number of patients with IPF will show subjective and objective improvement while receiving corticosteroid and cytotoxic therapy, and this has been shown to correlate with improved survival time. (25-28) Why select patients may respond to therapy with an improvement in clinical parameters of disease while the majority do not is unknown. Heterogeneity of histopathologic patterns has been shown to occur in some patients with IPF, with nonspecific interstitial pneumonia pattern or organizing pneumonia pattern at times accompanying the typical usual interstitial pneumonia pattern. (29,30) The presence of these mixed patterns on surgical lung biopsy may identify patients who are partially responsive to treatment. However, our current data suggest that this treatment-responsive group of patients has no impact on the overall survival curve of treated patients, confirming that at best they make up a small percentage of the overall population of patients with IPF. Importantly, the majority of patients treated with corticosteroid and cytotoxic therapy will have significant adverse effects from these medications. (31,32) Because the risk of adverse effects is high and any potential benefit is limited to a small subset of patients, physicians should carefully weigh the risks and benefits of using immunosuppressive therapy in individual patients. If the decision to use corticosteroid and a cytotoxic therapy is made, physicians should follow the ATS/ERS recommendations and assess patients frequently in order to minimize treatment-related adverse events. (1)
Why do patients with IPF respond so poorly to anti-inflammatory therapy--therapy that is effective for other forms of interstitial lung disease? The traditional pathophysiologic model of chronic and/or recurrent parenchymal inflammation leading to pathologic fibrosis may not apply to IPF. An alternative etiologic hypothesis has recently been advanced in which IPF represents abnormal wound healing in response to lung injury. (19-21) Abnormalities in alveolar epithelial cell repair, cytokine signaling (eg, transforming growth factor-[beta], interferon-[gamma]), fibroblast function, protease and antiprotease balance, and vascular remodeling have all been proposed. (21) Abnormal wound healing may allow an otherwise controlled injury to the lung to develop into a chronic, progressive fibroproliferative disorder in the absence of significant inflammation. New therapies for IPF that can attenuate this abnormal fibroproliferative response are sorely needed. (33,34)
In summary, we show in a large retrospective study of well-defined, matched patients with IPF that treatment with combined corticostereid and cyclophosphamide therapy does not alter survival. This finding further confirms the importance of investigating alternative therapies to improve the long-term outcome in these patients.
REFERENCES
(1) American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment; international consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000; 161:646-664
(2) Katzenstein ALA, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med 1998: 157: 1301-1315
(3) Bjoraker JA, Ryu JH, Edwin MK, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1998; 157:199-203
(4) Daniil ZD, Gilchrist FC, Nicholson AG, et al. A histologic pattern of nonspecific interstitial pneumonia is associated with a better prognosis than usual interstitial pneumonia in patients with cryptogenic fibrosing alveolitis. Am J Respir Crit Core Med 1999; 160:899-905
(5) Nicholson A, Colby TV, du Bois RM, et al. The prognostic significance of the histologic pattern of interstitial pneumonia in patients with the clinical entity of cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med 2000; 162:2213-2217
(6) American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2002;165:277-304
(7) Collard HR, King TE Jr. Demystifying idiopathic interstitial pneumonia. Arch Intern Med 2003; 163:17-29
(8) Johnson MA, Kwan S, Snell NJC, et al. Randomized controlled trial comparing prednisolone alone with cyclophosphamide and low those prednisolone in combination in cryptogenic fibrosing alveolitis. Thorax 1989; 44:280-288
(9) Raghu G, Depaso WJ, Cain K, et al. Azathioprine combined with prednisone in the treatment of idiopathic pulmonary fibrosis: a prospective,, double-blind randomized, placebo-controlled clinical trial. Am Rev Respir Dis 1991; 144:291-296
(10) Stack BHR, Choo-Kang YEJ, Heard BE. The prognosis of cryptogenic fibrosing alveolitis. Thorax 1972; 27:535-542
(11) Turner-Warwick M, Burrows B, Johnson A. Cryptogenic fibrosing alveolitis: response to corticosteroid treatment and its effect on survival. Thorax 1980; 35:593-599
(12) Izumi T, Nagai S, Kondo Y, et al. Ten-year follow-up of 222 patients with idiopathic pulmonary fibrosis [abstract]. Am Rev Respir Dis 1992; 145:A218
(13) Schwartz DA, Helmers RA, Galvin JR, et al. Determinants of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1994; 149:450-454
(14) Mapel DW, Hunt WC, Utton R, et al. Idiopathic pulmonary fibrosis: survival in population based and hospital based cohorts. Thorax 1998; 53:469-476
(15) Hubbard R, Johnston I, Britton J. Survival in patients with cryptogenic fibrosing alveolitis: a population-based cohort study. Chest 1998; 113:396-400
(16) Douglas WW, Ryu JH, Schroeder DR. Idiopathic pulmonary fibrosis: impact of oxygen and colchicine, prednisone, or no therapy on survival. Am J Respir Crit Care Med 2000; 161:1172-1178
(17) Riha RL, Duhig EE, Clarke BE, et al. Survival of patients with biopsy-proven usual interstitial pneumonia and nonspecific interstitial pneumonia. Eur Respir J 2002; 19:1114-1118
(18) Hunninghake GW, Zimmerman MB, Schwartz DA, et al. utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2001; 164: 193-196
(19) Pardo A, Selman M. Molecular mechanisms of pulmonary fibrosis. Front Biosci 2002; 7:d1743-d1761
(20) Selman M, Pardo A. Idiopathic pulmonary fibrosis: an epithelial/fibroblastic cross-talk disorder. Respir Res 2002; 3:3-10
(21) Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001; 134:136-151
(22) Meier-Sydow J, Rust M, Kronenberger H, et al. Long-term follow-up of lung function parameters in patients with idiopathic pulmonary fibrosis treated with prednisone and azathioprine or D-penicillamine. Prax Pneumol 1979; 33:680-688
(23) Raghu G, Mageto YN, Lockhart D, et al. The accuracy of the clinical diagnosis of new-onset idiopathic pulmonary fibrosis and other interstitial lung disease: a prospective study. Chest 1999; 116:1168-1174
(24) Flaherty KR, Thwaite EL, Kazerooni EA, et al. Radiological versus histological diagnosis in UIP and NSIP: survival implications. Thorax 2003; 58:143-148
(25) Collard HR, King TE Jr, Bucher-Bartelson B, et al. Changes in clinical and physiologic variables predict survival in idiopathic pulmona13t fibrosis. Am J Respir Crit Care Med 2003; 168:538-542
(26) Hanson D, Winterbauer RH, Kirtland SH, et al. changes in pulmonary function test results after 1 year of therapy as predictors of survival in patients with idiopathic pulmonary fibrosis. Chest 1995; 108:305-310
(27) Flaherty KR, Mumford JA, Murray S, et al. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med 2003; 168:543-548
(28) Latsi PI, du Bois RM, Nicholson AG, et al. Fibrotic idiopathic interstitial pneumonia: the prognostic value of longitudinal functional trends. Am J Respir Crit Care Med 2003; 168:531-537
(29) Katzenstein AL, Zisman DA, Litzky LA, et al. Usual interstitial pneumonia: histologic study of biopsy and explant specimens. Am J Surg Pathol 2002; 26:1567-1577
(30) Flaherty KR, Travis WD, Colby TV, et al. Histopathologic variability in usual and nonspecific interstitial pneumonias. Am J Respir Crit Care Med 2001; 164:1722-1727
(31) Douglas WW, Ryu JH, Swensen SJ, et al. Colchicine versus prednisone in the treatment of idiopathic pulmonary fibrosis: a randomized prospective study. Am J Respir Crit Care Med 1998; 158:220-225
(32) Flaherty KR, Toews GB, Lynch JP, et al. Steroids in idiopathic pulmonary fibrosis: a prospective assessment of adverse reactions, response to therapy, and survival. Am J Med 2001; 110:278-282
(33) Ziesche R, Hofbauer E, Wittmann K, et al. A preliminary study of long-term treatment with interferon [gamma]-lb and low-dose prednisolone in patients with idiopathic pulmonary fibrosis. N Engl J Med 1999; 341:1264-1269
(34) Raghu G, Johnson WC, Lockhart D, et al. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open-label phase II study. Am J Respir Crit Care Med 1999; 159:1061-1069
* From the Department of Medicine (Drs. Collard and Schwarz), Division of Pulmonary Sciences and Critical Care Medicine, University of Colorado Health Sciences Center, Denver, CO; Department of Internal Medicine (Drs. Ryu and Douglas), Division of Pulmonary and Critical Care Medicine, Mayo Clinic, Rochester, MN; National Jewish Medical and Research Center (Dr. Brown), Denver, CO; Departments of Preventive Medicine and Biometrics (Dr. Curran-Everett), University of Colorado Health Sciences Center, Denver, CO; and Department of Medicine (Dr. King), San Francisco General Hospital, University of California at San Francisco, San Francisco, CA.
Supported by National Institutes of Health grants SCOR HL6767 and NHLBI HL07085.
Manuscript received July 22, 2003; revision accepted January 30, 2004.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (e-mail: permissions@chestnet.org).
Correspondence to: Kevin K. Brown, MD, FCCP, 1400 Jackson St, Boom F107, Denver, CO 80206; e-mail: brownk@njc.org
COPYRIGHT 2004 American College of Chest Physicians
COPYRIGHT 2004 Gale Group