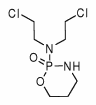
A GREAT DEAL of evidence that demonstrates a functional relationship between the central nervous system and the immune system has been accumulated over the past 25 years. Dramatic evidence of an interactive network between these systems is seen in demonstrations of learned--that is, conditioned--alterations of immunologic reactivity.
Pavlov's (1928) work provides a paradigm for the conditioning of behavioral and physiological responses through the pairing of stimuli. Initially, a neutral stimulus (the conditioned stimulus, or CS) is paired with an unconditioned stimulus (the UCS) that naturally elicits a particular response (the unconditioned response, or UCR). As a consequence of repeated associations between the CS and the UCS, the former acquires the ability to elicit a conditioned response (the CR) that is related to that evoked by the UCS. A clinically relevant example of the ability of a former neutral stimulus to elicit a reflexive response, in the absence of the stimulus that naturally evokes it, is illustrated by Mackenzie's (1896) intentional provocation of an allergic reaction by presentation of an artificial rose to an allergic patient--the so-called "rose cold."
Ader and Cohen began modern studies of conditioned alterations of immunologic reactivity in 1975 with immunosuppressive responses. Rats drank a saccharin solution immediately before the injection of cyclophosphamide, an immunosuppressive drug that also has aversive gastrointestinal side effects. Following this pairing, rats avoided drinking the saccharin solution. This avoidance phenomenon is called conditioned taste aversion (Garcia, Kimeldorf, & Koelling, 1955). However, rats exposed to the saccharin solution also showed significant conditioned immunosuppression. This experiment marked the beginning of the field of psychoneuroimmunology.
The work of O'Reilly and Exon (1986) is another example of research conducted in this field. These researchers paired a saccharin taste CS with the immunosuppressing UCS, cyclophosphamide. One of cyclophosphamide's natural effects is the reduction of natural killer-cell activity. Natural killer-cells are one of an organism's first defenses against the development of malignant tumors. When they find a cell that has been infected with a virus or one that has become cancerous, they engulf and destroy it (Carlson, 1998). When O'Reilly and Exon presented saccharin to their rat subjects, it resulted in a conditioned taste aversion as well as a conditioned reduction in natural killer-cell cytotoxicity.
The findings of Ader and Cohen (1975) and O'Reilly and Exon (1986) are examples of the classical conditioning phenomenon of conditioned excitation. A conditioned excitor (CS+), such as the taste of saccharin in the aforementioned studies, signals the occurrence of a particular UCS, namely, cyclophosphamide. A conditioned inhibitor (CS-), on the other hand, signals the absence of that UCS. Theoretically, it should produce a physiological effect that is directly opposite to that of a CS+. Although there have been discriminative conditioning experiments of immunomodulation (Buske-Kirschbaum, Kirschbaum, Stierle, Jabaij, & Heilhammer, 1994), to date, there have been no demonstrations of conditioned inhibition using an immunosuppressive drug such as cyclophosphamide.
The purpose of the present experiment was to determine whether a stimulus placed in a negative relationship with cyclophosphamide would function as a conditioned inhibitor in a taste aversion paradigm. In the study by O'Reilly and Exon (1986), the saccharin CS+ became aversive. That is, in a two-bottle--saccharin-water preference test, subjects should prefer water to saccharin. A CS-, on the other hand, because it signals the absence of cyclophosphamide and its gastrointestinal upset, should become a preferred taste (compared with water) in a two-bottle preference test. Best (1975) and Lambert et al. (1989) clearly demonstrated that a taste that had come to signal the absence of illness would be preferred over a taste that had not acquired such conditioned illness-inhibitory properties.
Successful demonstration of conditioned inhibition using an immunosuppressive UCS would demonstrate further the generality of this phenomenon within the conditioned taste-aversion framework. In addition, such a demonstration would suggest that one might use a CS- to actively inhibit conditioned and unconditioned immunosuppressive responses. Given that the reduction of natural killer-cell activity is associated with stress (Kiecolt-Glaser, Garner, Speicher, Penn, & Glaser, 1984) and depression (Irwin, Smith, & Gillen, 1987), such a finding would have obvious potential clinical ramifications.
Method
Subjects
Thirty male, 6-week-old, Sprague-Dawley rats served as subjects. They weighed approximately 150 g at the start of the experiment. They were housed individually and were maintained on a 12-hr light--dark cycle. They were allowed free access to food and water for 1 week after arriving at the vivarium.
Stimuli
The excitatory conditioned stimulus (CS+) was a 3% solution of sodium saccharin. The inhibitory conditioned stimulus (CS-) was a solution of McCormick's vanilla extract (1 ml extract to 30 ml water). The UCS was a subcutaneous injection of 50 mg/kg cyclophosphamide (Sigma Chemical Co., St Louis, MO) in a volume of 1.0 cc sterile water. The neutral stimulus used in the two-bottle tests was tap water.
Design
Sixteen rats were randomly assigned to an experimental group, and 14 rats to a control group. The experimental group received two types of trials. On some trials, they were exposed to the saccharin-CS+ followed by the UCS. On other trials, however, the CS+ was presented in conjunction with the vanilla-CS- and no UCS followed. This is called an A+, AX- conditioning paradigm, where A+ is the excitatory stimulus and X--the inhibitory stimulus. The control group was presented (or not presented) with the UCS in the absence of the taste stimuli.
Procedure
After the 1-week period of adaptation to the laboratory environment (which included handling and weighing), the rats were placed on a water-deprivation schedule that permitted them 15 min/day of water in their home cages once every 23 hr. This water-deprivation schedule was in effect for 5 days prior to the start of the experiment. Two types of taste tests were used. The first was a single-bottle test in which rats were exposed to a bottle containing 100 ml of either the vanilla solution or the saccharin solution. The duration of the pretest--single-bottle exposure was 15 min, and that of the posttest exposure was 30 min. The second was a postconditioning--two-bottle choice test that took place over a 2-day period. On the 1st day, rats were exposed to two adjacent bottles containing 100 ml water and 100 ml saccharin. On the 2nd day, the choice was between two adjacent bottles containing 100 ml water and 100 ml vanilla, respectively.
Phase I was the initial single-bottle taste test. On Days 1 and 2, all rats received a 15-min exposure in their home cages to single bottles containing the saccharin and vanilla solutions, respectively. The amount of fluid consumed was the dependent measure.
The conditioning took place in Phase 2.
Experimental group. There were two CS+ and two CS- trials. On Days 3 and 10, the rats received a bottle containing the saccharin solution during their 15-min drinking period. Immediately afterward, they received a subcutaneous injection of 50 mg/kg cyclophosphamide in a volume of 1.0 cc sterile water.
On Days 17 and 24, the rats received a bottle containing the saccharin solution for the first 2 mm of their 15-min drinking period. They received a bottle of the vanilla solution during the remaining 13 min. Immediately afterward, they received a subcutaneous injection of 1.0 cc of sterile water.
UCS-only control group. On Days 3 and 10, the rats received a subcutaneous injection of 50 mg/kg cyclophosphamide in a volume of 1.0 cc sterile water. Two hr later, they received a bottle containing plain tap water during a 15-min drinking period. On Days 17 and 24, rats received a subcutaneous injection of 1.0 cc of sterile water. Two hr later, they received one bottle containing plain tap water during their 15-mm drinking period.
The postconditioning taste tests were conducted in Phase 3.
Single-bottle test. On Day 25, half of the rats in each group were randomly assigned to the following conditions:
* Condition 1. Rats received a single bottle containing 100 ml of the saccharin solution during a 30-min drinking period; and
* Condition 2. Rats received a single bottle containing 100 ml of the vanilla solution during a 30-min drinking period.
We predicted that the experimental group would consume less of the saccharin solution and more of the vanilla solution than would the control group.
Two-bottle choice tests. On Days 26 and 27, during their 15-min drinking period, Condition 1 rats were presented with two adjacent bottles containing 100 ml of water and the saccharin solution, respectively. For Condition 2 rats, these bottles contained water and vanilla solution, respectively. The percentage of solution consumed was calculated according to the following formula: [flavor-solution consumed/(flavor-solution consumed + water consumed)] x 100.
We predicted that the percentage of saccharin solution consumed by the experimental group would be less than that consumed by the control group. We also predicted that the percentage of vanilla solution consumed by the experimental group would be greater than that consumed by the control group. The two-bottle choice test preference meets Best's (1975) definition of conditioned inhibition.
Results and Discussion
A Mann-Whitney U test applied to the ranked volume-consumed data for saccharin and vanilla during the initial single-bottle taste tests revealed no differences between the groups in the consumption of saccharin or vanilla. The mean volume of saccharin consumed by the experimental group was 15.8 ml. That consumed by the control group was 15.5 ml (U = 102.5, p > .05). The mean volume of vanilla solution consumed by the experimental group was 14.0 ml; that consumed by the control group was 13.9 ml (U = 103, p > .05).
Mann-Whitney U tests applied to the ranked volume-consumed data for saccharin during the 30-min, postconditioning-single-bottle test revealed a significant difference between the groups, U = 8, p < .05 (one-tailed). The mean volume of saccharin consumed by the experimental group was 57.75 ml; the mean for the control group was 70.57 ml. The overall increase in consumption for both groups from pretest to posttest is a reflection of the increase in posttest exposure time (15 min in the pretest to 30 min in the posttest) to the saccharin solution. Nevertheless, the 13-ml difference in saccharin consumption between the two groups of fluid-deprived rats indicates the development of an aversion to the taste of saccharin in the experimental group compared with the control group.
A repeated measures analysis of variance (ANOVA) conducted on the pre-post conditioning measures of fluid consumption for the single-bottle tests (see Table 1) showed a significant pre-post test interaction, F(1, 26) = 6.64, p < .02. Fisher's least-significant difference tests indicated that the experimental group consumed less saccharin (57.8 ml) than did the control group (70.6 ml), but there was no significant between-groups difference in vanilla consumption. The mean volume of vanilla consumed by the experimental group was 77.13 ml; that consumed by the control group was 74.14 ml. This result did not support the prediction of greater vanilla consumption in the experimental group in the single-bottle test.
Figure 1 depicts the percentage of saccharin and vanilla consumption, relative to water, for the two groups during the postconditioning-two-bottle preference tests. A taste aversion to saccharin was conditioned in the experimental group as shown by a lower percentage-consumption of saccharin (M = 25.5) compared with that of the control group (M = 36.9). A Mann-Whitney U test applied to the ranked percentage-consumption data revealed this difference to be significant, U = 65.5, p < .05 (one-tailed).
As may also be seen, the percentage of vanilla solution consumed by the experimental group (M = 58.2) was greater than that of the control group (M = 45.3). A Mann-Whitney U test applied to the ranked percentage-consumption data revealed this difference to be significant, U = 67, p < .05 (one-tailed), indicating the development of a taste preference (with respect to water) in the former group, compared with the latter. These two-bottle choice test preferences meet Best's (1975) definition of conditioned inhibition and indicate that the vanilla solution had acquired conditioned inhibitory properties in the experimental group relative to the control group.
Konorski (1967) asserted that conditioned inhibitors elicit a state that is opposite to that elicited by unconditioned stimuli. Cyclophosphamide elicits not only gastrointestinal upset but also suppression of the natural killer-cell response (O'Reilly & Exon, 1986). Presentation of a conditioned inhibitor, theoretically, should produce the opposite effects--that is, no gastrointestinal upset and enhancement of the natural killer-cell response. The preference shown by the experimental group for the vanilla solution in the two-bottle test is indicative of the former. Future research in which natural killer-cell activity is assayed is necessary to test the latter prediction. Given the role of suppressed natural killer-cell activity in stressed (Kiecolt-Glaser et al., 1984, 1986) and depressed individuals (Irwin et al., 1987), it is imperative that further research on learned aspects (e.g., conditioned inhibitory) of natural killer-cell activity be conducted.
[FIGURE 1 OMITTED]
Manuscript received August 2, 1999
Revision accepted for publication March 6, 2001
REFERENCES
Ader, R., & Cohen, N. (1975). Behaviorally conditioned immunosuppression. Psychosomatic Medicine, 37, 333-340.
Best, M. R. (1975). Conditioned and latent inhibition in taste aversion learning. Journal of Experimental Psychology: Animal Behavior Processes, 1, 97-113.
Buske-Kirschbaum, A., Kirschbaum, C., Stierle, H., Jabaij, L., & Heilhammer, D. (1994). Conditioned manipulation of natural killer (NK) cells in humans using a discriminative learning protocol. Biological Psychology, 38, 143-155.
Carlson, N. R. (1998). Physiology of behavior (6th ed.). Boston: Allyn & Bacon.
Garcia, J., Kimeldorf, D. J., & Koelling, R. (1955). Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science, 122, 157-158.
Irwin, M., Smith, T. L., & Gillen, J. C. (1987). Low natural killer cytotoxicity in major depression. Life Sciences, 41, 2127-2133.
Kiecolt-Glaser, J. K., Garner, W., Speicher, C. E., Penn, G., & Glaser, R. (1984). Psychosocial modifiers of immunocompetence in medical students. Psychosomatic Medicine, 46, 7-14.
Kiecolt-Glaser, J. K., Glaser, R., Strain, E., Stout, J., Tarr, K., Holliday, J., & Speicher, C. E. (1986). Modulation of cellular immunity in medical students. Journal of Behavioral Medicine, 9, 5-21.
Konorski, J. (1967). Integrative activity of the brain: An interdisciplinary approach. Chicago: Chicago University Press.
Lambert, J. V., Barber, R. M., Carpenito, P., Cianfrani, M., Mendez, B., & Potosnak, L. C. (1989). Conditioned inhibition of rotation-induced taste aversion. Animal Learning & Behavior, 17, 457-467.
Mackenzie, J. N. (1896). The production of the so-called "rose cold" by means of an artificial rose. American Journal of Medical Science, 91, 45-57.
O'Reilly, C. A., & Exon, J. H. (1986). Cyclophosphamide-conditioned suppression of the natural killer cell response in rats. Physiology & Behavior, 37, 759-764.
Pavlov, I. P. (1928). Lectures on conditioned reflexes. New York: International Publishers.
The authors thank Catherine E. Lambert for the preparation and injection of the cyclophosphamide, and Suzanne K. Murphy for her consultation on the project. They also thank Nancy Davis for animal care.
This research was made possible through in-house support by the College of Arts and Sciences of the University of the Sciences in Philadelphia. The data were presented at the annual meeting of the Eastern Psychological Association, Baltimore, MD, March 23-25, 2000.
Address correspondence to Joseph V. Lambert, Department of Social Sciences, University of the Sciences in Philadelphia, 600 S. 43rd Street, Philadelphia, PA 19104 (j.lamber@usip.edu); or Wayne G. Whitehouse, Department of Psychology, Weiss Hall, Temple University, Philadelphia, PA 19122 (wwhiteho@thundar.temple.edu).
COPYRIGHT 2002 Heldref Publications
COPYRIGHT 2002 Gale Group