Pharmaceutical giant Andrx Corp. put the brakes on its attempt to develop and market brand name drugs after the unit it set up two years ago to lead the effort posted millions of dollars in losses last year. Now company executives say Andrx will go back to its profitable, core, generic drug business.
Brand name pharmaceuticals held the promise of higher profits--73 percent gross margins versus 47 percent for generic drugs, according to the Andrx annual report. Experts estimate the company spent millions of dollars to set up and maintain the brand name unit, and the Andrx board of directors hired a new CEO to lead the effort. But the profits never materialized, at least not with any consistency.
The Plantation-based firm's brand name business posted a $31 million operating loss for the first nine months of 2004, and a $50 million loss for the same period in 2003, according to the company's third quarter 10-Q filing with the Securities and Exchange Commission. This January Andrx announced it would sell off that part of its business.
"Our core competencies lie in controlled-release technologies and our distribution operation," Andrx president Angelo Malahias says. "Our controlled-release technologies allow us to genericize difficult-to-replicate products."
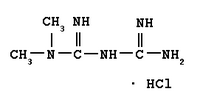
Controlled-release technologies slowly release a dose of medication into a patient's body at timed intervals. Andrx manufactures one of the most popular of those drugs: a generic version of Claritin-D 24 that releases a decongestant and an antihistamine, giving a patient relief from cold or allergy symptoms throughout a 24-hour period.
Andrx faces limited competition in the controlled-release drug business because the medications are difficult to engineer and manufacture, Malahias says. Generics generated $105 million of operating income for Andrx during the first nine months of 2004. The business represented $303 million of the company's $855 million in revenue during that period. Andrx has manufactured generic versions of popular drugs since the company launched in 1992.
"We are strong in generics," Malahias says. "We will sell 30 generic products and have another 30 products pending with the USFDA [Food and Drug Administration]. We spent more than $40 million on research and development in 2004 and expect to see a significant return on this investment."
Malahias would not elaborate on which products are pending or their prospects, but he did say the company plans to boost its distribution units, Anda and VIP, which combined are the nation's No. 4 distributor of generic pharmaceuticals. "Right now we are distributing Andrx generic products along with 7,000 generic products manufactured by other companies," he says. "We distribute primarily to independent pharmacies and non-warehouse pharmacy chains. We are the only generic drug manufacturer in the US with our own distribution operation."
Distribution accounted for $495 million of revenue during the first nine months of 2004, and generated $37 million in operating income.
[ILLUSTRATION OMITTED]
Hours before Andrx announced it was getting out of the brand business, an equities analyst at Advest raised its stock rating from "neutral" to "buy," citing the possibility the company would spin off the brand business.
Jeffrey Long-McGie, an analyst who follows Andrx for ThinkEquity Partners, says the move is a positive one for the long-term. "I think Andrx is positioned well and will do well in the long term. They are going back to their core competencies and I think this is a good thing," he says.
Even so, the generics market has its pit-falls. Pharmaceutical makers normally invest billions of dollars to research and develop new drugs, which undergo lengthy, expensive trials before regulators approve them for sale to the public. That is one reason generic drug manufacturers like Andrx are a thorn in the side of large drug companies. Andrx can receive federal approval for its drugs at a fraction of the cost of a brand name drug maker. And once a patent on a brand name medicine expires, generic drug makers have the right to manufacture them as soon as they get regulator approval.
Generic drug manufacturers do not just copy. Some develop different delivery mechanisms to make drugs more effective--a process that can incur risk if regulators find that the changes infringe on a drug's patent.
"Generic companies don't have to do a lot of expensive research and don't have to go through the clinical trials," says Matthew Seamon, an assistant professor at Davie-based Nova Southeastern University's College of Pharmacy. "[Instead] they are constantly in court rooms trying to battle patents."
Malahias concurs, and adds that losing a patent-infringement lawsuit can be costly. "This a very challenging field, legally," he says. "In almost every situation, we face a lawsuit from the original producer. Right now, our company is valued at $1.6 billion but it has been valued as high as $6 billion. The reason we lost value is because we lost a patent infringement case on Prilosec, manufactured by AstraZeneca."
In that case, Andrx was found to have infringed on AstraZeneca's patent for the stomach medicine Prilosec, and was barred from bringing a generic version of the "little purple pill" to market.
Andrx' own brand name drugs, Alto-prev for cholesterol control (launched nearly three years ago) and Fortamet for type-two diabetes (launched in May 2004), are now on the selling block.
Malahias declined to say if selling the brand name business would mean job cuts at its corporate headquarters in Plantation, distribution headquarters in Weston or generic manufacturing facility in Davie. Company-wide, Andrx has 2,100 employees. The company had a public stock offering in June 1996 and raised $120 million in capital to launch its first generic product, Diltia XT, in 1997.
COPYRIGHT 2005 CEO Publishing Group, Inc.
COPYRIGHT 2005 Gale Group