Guilford Pharmaceuticals Inc., (NASDAQ: GLFD) announced today that
the Oncologic Drugs Advisory Committee (ODAC) voted 8 to 5 that
GLIADEL(R) Wafer (polifeprosan 20 with carmustine implant) provides
clinical benefit with an acceptable safety profile for the treatment
of patients with newly diagnosed malignant glioma. Some committee
members, however, expressed concerns about the size of the study, the
analytical methods and the pathological diagnosis. While the FDA is
not bound by the recommendation, it traditionally follows the
Committee's advice.
"The positive outcome of the FDA Advisory Committee meeting is an
important step forward in our efforts to bring the benefits of
GLIADEL(R) Wafer to more patients," said Craig R. Smith, M.D.,
President and Chief Executive Officer of Guilford. "We will continue
to work closely with the FDA to help them expeditiously complete
their action on our sNDA." Clinical Trial Design
The Committee based their recommendation to approve an expanded label
for GLIADEL(R) Wafer on a randomized, double-blind,
placebo-controlled Phase III clinical trial, which was conducted in
240 eligible men and women at 38 study centers in 14 countries.
Beginning in December 1997, patients were enrolled and randomly
assigned to receive either GLIADEL(R) Wafer or placebo wafers, in
addition to receiving standard therapy for malignant glioma, which
included surgery and radiation.
The primary endpoint in the study was overall survival at 12 months.
Depending on the date of enrollment in the study, patients were
followed for a minimum of 12 months and a maximum of 30 months.
Secondary endpoints included measures of neurological outcome and
disease progression.
Clinical Trial Results
The Phase III study results demonstrated about a 30 percent improvement
in survival rates and neurological symptoms in patients who received
GLIADEL(R) Wafer therapy. At one year, 59 percent of patients given
GLIADEL(R) Wafer were still alive compared to 48 percent of those
given placebo. A secondary endpoint in the study - improvement in
neurological symptoms - also demonstrated a significant benefit in
the GLIADEL(R) Wafer treatment group.
"Implanted at the time of initial surgery, GLIADEL(R) Wafer offers
patients the benefits of localized chemotherapy without the
devastating side effects of systemic treatments, while offering the
hope of extended survival and delayed onset of neurological
deterioration," said Alan Hamilton, MD, Chief, Division of
Neurosurgery, University of Arizona Health Sciences Center.
GLIADEL(R) Wafers have generally been well tolerated. However,
GLIADEL(R) Wafer contains carmustine and should not be given to
patients who are allergic to carmustine. Carmustine can also cause
fetal harm when administered to a pregnant woman. Patients
undergoing surgery for malignant brain cancer and implantation of
GLIADEL(R) Wafer should be monitored closely for the following known
complications of surgery including: seizures, intracranial
infections, abnormal wound healing, and brain edema. Each of these
complications has been associated with GLIADEL(R) Wafer treatment.
About GLIADEL(R) Wafer
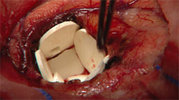
GLIADEL(R) Wafer is a small, white dime-sized wafer made of a
biodegradable polymer that contains the cancer chemotherapeutic drug,
carmustine or BCNU. Up to eight GLIADEL(R) Wafers can be implanted
in the cavity created when a brain tumor is surgically removed.
There, the wafers slowly dissolve over a period of 2 to 3 weeks,
delivering chemotherapy directly to the tumor site in high
concentrations, while minimizing drug exposure to other areas of the
body.
GLIADEL(R) Wafer received marketing approval from the FDA in September
1996 to treat recurrent glioblastoma multiforme (GBM), an aggressive
form of malignant brain cancer. Prior to the availability of
GLIADEL(R) Wafer, standard of care for GBM patients was removal of
the tumor followed by radiation and systemic chemotherapy.
About GBM
Primary brain tumors are tumors that originate in the brain.
Astrocytoma, medulloblastoma and GBM are examples of primary brain
tumors. GBM is considered one of the most rapidly progressive and
universally fatal of all cancers. According to the American Brain
Tumor Association, there are approximately 17,500 primary brain
tumors diagnosed in the United States each year. Of these, GBM is
the most common type. Even with aggressive conventional treatment
the typical patient lives less than one year after diagnosis.
Guilford Pharmaceuticals Inc. is a global, fully-integrated
pharmaceutical company targeting the neurological, surgical and
critical care markets. The Company's mission is to develop
proprietary biopolymer-based therapeutics for surgeons and novel
pharmaceutical products for the diagnosis and treatment of
neurological disorders.
Conference Call
Guilford will host a conference call to review the ODAC Panel Meeting.
The conference call will take place at 9:00 a.m. E.T. on Friday,
December 7, 2001. The dial in number is (212) 346-7427.
Conference Call Replay
An audio replay of the conference call will be available for 24 hours
from 11:00 a.m. ET on December 7, 2001 through 11:00 a.m. December 8,
2001. To access the replay, dial 1-800-633-8284, (int'l callers
858-812-6440), then dial reservation number 20078296.
For more information about GLIADEL(R) Wafer, visit www.gliadel.com.
For more information about Guilford Pharmaceuticals, visit
www.guilfordpharm.com
This press release contains forward-looking statements that involve
risks and uncertainties, including those described in the section
entitled "Risk Factors" contained in the Company's Registration
Statement on Form S-3 filed with the SEC on June 21, 2001 (File No.
333-63576). Among other things, there can be no assurance that the
FDA will follow ODAC's recommendation and approve GLIADEL(R) Wafer
for initial therapy in patients newly diagnosed with malignant
glioma.