The antiparasitic animal drug ivermectin was approved by FDA to treat two human parasitic diseases, strongyloidiasis and onchocerciasis.
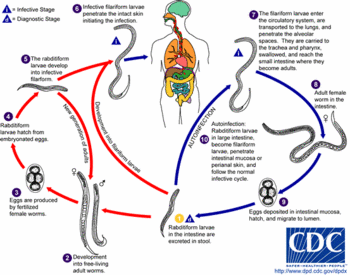
Strongyloidiasis is common in many tropical countries and is occasionally acquired in some areas of the United States. Infection is usually confined to the small intestine. It can persist for many years, causing abdominal pain, diarrhea, and elevated levels of white blood cells. In people with weakened immune systems, the infection can spread through the body and be fatal.
Onchocerciasis, commonly known as river blindness, is most often found in Africa and South and Central America. The parasite is transmitted by the bite of a black fly, which deposits immature forms of the parasite under the skin. There, the worms mature and the adult females produce more larvae. The immature worms migrate under the skin, causing intense itching. They can also migrate to the eyes, causing inflammation and blindness.
Ivermectin is better tolerated than other drugs approved to treat these conditions, and it is more effective than the previously approved treatment for onchocerciasis. In clinical studies, a single dose of ivermectin cured between 64 and 100 percent of patients with strongyloidiasis who had normal immune systems. In studies of onchocerciasis, a single dose of the drug reduced the number of larvae under the skin an average of 83 percent at three days and 99.5 percent at three months. Adverse reactions to the drug differ depending on which disease is being treated but can include skin rashes, itching, dizziness, diarrhea, and nausea.
Merck & Co. Inc., of West Point, Pa., sells ivermectin in the United States under the trade name Stromectol. (Elsewhere it is marketed as Mectizan.) Before its approval on Nov. 25, 1996, ivermectin had been available here for limited human use as an investigational drug.
Ivermectin has been used worldwide since 1987 to treat river blindness. More than 5.2 million people have received the drug through the World Health Organization's Onchocerciasis Control Program and Merck's Mectizan Donation Program.
(See also "Treating Tropical Diseases" in the January-February 1997 FDA Consumer.)
COPYRIGHT 1997 U.S. Government Printing Office
COPYRIGHT 2004 Gale Group