Definition
Threadworm infection is an intestinal disease, which occasionally spreads to the skin, caused by a type of parasitic roundworm (helminth). In untreated patients, the disease has a high rate of reinfection caused by worms already present in the body. This type of disease recurrence is called autoinfection. Because of autoinfection, threadworms can remain inside humans for as long as 45 years after the initial infestation.
Description
Threadworm infection, which is also called strongyloidiasis, occurs in most countries of the world but is natural to (endemic in) tropical and subtropical climates. Strongyloidiasis is less common than other parasitic infections but may affect as much as 25% of the population in some developing countries. In the United States, threadworm infection is most likely to be found among immigrants; returning travelers or military personnel; people who live in parts of Appalachia and the southeastern states; and persons in homes for the retarded and similar institutions.
Human beings are universally susceptible to threadworm infection, although adults and older children are at greater risk of infection than younger children. The disease does not confer immunity. In addition to humans, threadworms can infect dogs, cats, horses, pigs, rats, and monkeys.
Causes & symptoms
Threadworm infection is caused by Strongyloides stercoralis, a roundworm that lives in soil and can survive there for several generations. Mature threadworms may grow as long as 1-2 in (2.5-5 cm). The larvae have two stages in their life cycle: a rod-shaped (rhabdoid) first stage, which is not infective; and a threadlike (filariform) stage, in which the larvae can penetrate intact human skin and internal tissues.
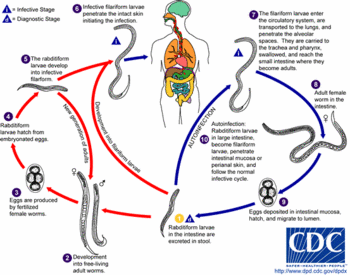
The infection is most commonly transmitted when a person comes into contact--usually by walking barefoot--with soil containing S. stercoralis larvae in their filariform stage. The threadlike larvae penetrate the skin, enter the lymphatic system, and are carried by the blood to the lungs. Once in the lungs, the larvae burst out of the capillaries into the patient's main respiratory system. They migrate upwards--usually without symptoms--to the patient's throat, where they are swallowed and carried down into the digestive tract. The filariform larvae settle in the small intestine. They mature into adults that deposit eggs that hatch--usually in the intestines-- into noninfectious rhabdoid larvae. The rhabdoid larvae then migrate into the patient's large intestine and are excreted in the feces. The time from initial penetration of the skin to excretion is 17-28 days. The rhabdoid larvae metamorphose into the infective filariform stage in the soil.
Threadworms are unique among human parasites in having both free-living and parasitic forms. In the free-living life cycle, some rhabdoid larvae develop into adult worms that live in contaminated soil and produce eggs that hatch into new rhabdoid larvae. The adult worms may live as long as five years.
The signs and symptoms of threadworm infection vary according to the stage of the disease as the larvae migrate throughout the body. Patients who suffer from autoinfection may have chronic or intermittent symptoms for years after they are first infected.
Skin
The filariform larvae usually enter the body through the skin of the feet. There may be swelling, itching, and hives at the point of entry that may be confused with insect bites. Patients with chronic threadworm infection may also develop an itchy rash on their buttocks, thighs, or abdomen.
Digestive tract
Although some patients may notice only mild diarrhea and cramps, others may have fever, nausea, vomiting, general weakness, and blood or mucus in their stools. The pain may mimic a stomach ulcer.
Throat and lungs
When the larvae migrate to the lungs and air passages, the patient may have symptoms ranging from a simple dry cough to fever, difficulty breathing, and coughing up blood or pus.
Hyperinfection syndrome
Hyperinfection syndrome is a potentially fatal set of complications resulting from the spread of filariform larvae to the lungs and other organ systems. It can include inflammation of the heart tissue, stomach ulcers, perforation of the intestines, blood poisoning, meningitis, shock, and eventual death. Hyperinfection syndrome is most likely to occur in patients with immune disorders or malnutrition, or in those taking anti-inflammatory corticosteroid (anti-inflammatory) medications. It has been reported in only a few AIDS patients.
Autoinfection
Threadworm autoinfection in humans follows two patterns. In internal autoinfection, some rhabdoid larvae in the lower bowel develop into filariform larvae that enter the bloodstream from the intestines and migrate to the lungs. In external autoinfection, the skin around the patient's anus is infected by larvae in the feces.
Diagnosis
The doctor is likely to consider a diagnosis of threadworm infection when a patient has the symptoms described earlier and a history of travel or military service in areas where the disease is endemic. A definite diagnosis is made by finding rhabdoid or filariform larvae in the patient's body fluids. The larvae may be found in fresh stool specimens or in mucus coughed up when the infection has reached the lungs. Because the larvae cannot be detected in the stools of 25% of infected patients, the string test is often performed to confirm the diagnosis. In this test, the patient swallows a weighted string which is withdrawn after four hours. The digestive juices absorbed by the string are then examined for the presence of threadworm larvae.
Doctors can also use blood tests and diagnostic imaging to support the diagnosis. Between 85% and 95% of patients with threadworm infections will have a measurable level of antibodies in their blood, even though these antibodies do not prevent the disease from spreading. In addition, patients with severe infections often have unusually high levels of white cells in their blood. X rays of the intestines or the chest often help in locating specific areas of inflamed or ulcerated tissue.
Treatment
Threadworm infections are treated with medications. The drugs most often given are ivermectin, thiabendazole (Mintezol), and albendazole. Ivermectin is generally preferred because it has fewer side effects than thiabendazole. These drugs, which are taken by mouth over a period of 2-7 days, work by preventing the development of eggs and new larvae. Patients with severe infections should be given protein replacement, blood transfusions, and fluids to replace losses from nausea, vomiting, and diarrhea.
Patients who are taking corticosteroids should be carefully evaluated if they have symptoms of threadworm infection, because these medications encourage the development of hyperinfection syndrome.
Prognosis
The prognosis for complete recovery is good for most patients, except those with hyperinfection syndrome or severe protein loss.
Prevention
There is no effective immunization against threadworm infection. Prevention of the disease requires careful attention to personal and institutional hygiene in endemic areas, including handwashing after defecating and before handling food. Other precautions include wearing shoes when visiting countries with high rates of threadworm infection, and monitoring close contacts of patients for signs of infection.
Key Terms
- Antibody
- A protein molecule produced by the immune system that is specific to a disease agent, such as threadworm larvae. The severity of a patient's infection can be measured from the level of antibody in the blood.
- Autoinfection
- An infection caused by a disease agent that is already present in the body.
- Corticosteroid
- A class of drugs based on hormones formed in the adrenal gland, used to reduce inflammation. They increase the likelihood of hyperinfection syndrome in patients with threadworm infection.
- Endemic
- Natural to or characteristic of a particular place, population, or climate. Threadworm infections are endemic in the tropics.
- Filariform
- Threadlike in appearance, like the infectious stage of the threadworm larva.
- Helminth
- A type of parasitic worm. Threadworms belong to a subcategory of helminths called nematodes, or roundworms.
- Hyperinfection syndrome
- A condition of massive infection in which threadworm larvae multiply rapidly and spread throughout the body. It is usually associated with damage to the immune system, the use of steroid medications, or malnutrition.
- Rhabdoid
- Rod- or wand-shaped, like the first stage of the threadworm larva.
- String test
- A test performed to diagnose threadworm infection. The patient is asked to swallow a weighted string that absorbs stomach juices, which can be analyzed for the presence of threadworm larvae.
Further Reading
For Your Information
Books
- Genta, Robert M. "Strongyloidiasis." In Encyclopedia of Immunology, vol. III, edited by Ivan M. Roitt and Peter J. Delves. London: Academic Press, 1992.
- Goldsmith, Robert S., MD, "Infectious Diseases: Protozoal & Helminthic." In Current Medical Diagnosis & Treatment 1998, edited by Lawrence M. Tierney, Jr. et al. Stamford, CT: Appleton & Lange, 1998.
- "Infectious Disease: Parasitic Infections." In The Merck Manual of Diagnosis and Therapy, vol. I, edited by Robert Berkow, et al. Rahway, NJ: Merck Research Laboratories, 1992.
- McCarthy, James S., and Thomas B. Nutman, "Parasitic Diseases of the Skin." In Conn's Current Therapy, edited by Robert E. Rakel. Philadelphia: W. B. Saunders Company, 1997.
- Phillips, Elizabeth, and Jay S. Keystone. "Intestinal Parasites." In Conn's Current Therapy, edited by Robert E. Rakel. Philadelphia: W. B. Saunders Company, 1997.
- Weinberg, Adriana, and Myron J. Levin. "Infections: Parasitic & Mycotic." In Current Pediatric Diagnosis & Treatment, edited by William W. Hay, Jr., et al. Stamford, CT: Appleton & Lange, 1997.
Gale Encyclopedia of Medicine. Gale Research, 1999.